

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50136071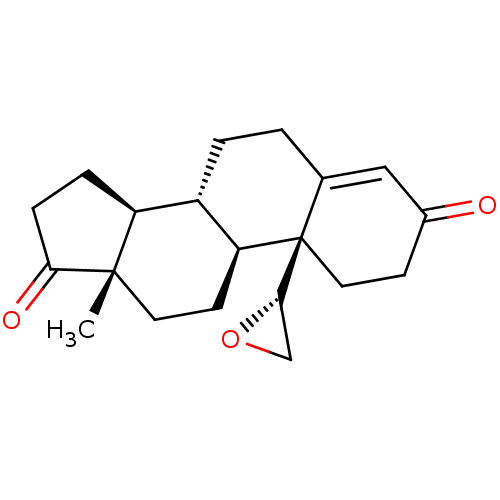 (CHEMBL3753593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM50398334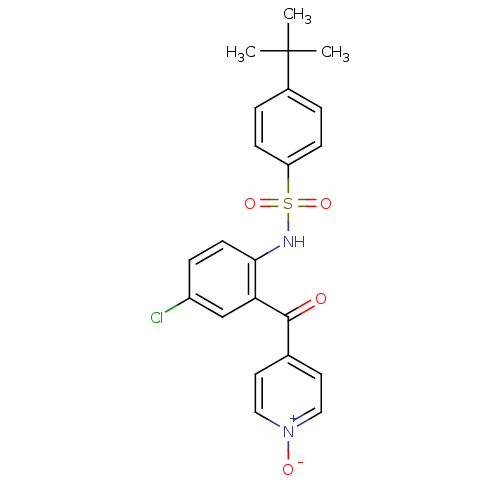 (CHEMBL2178578) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136057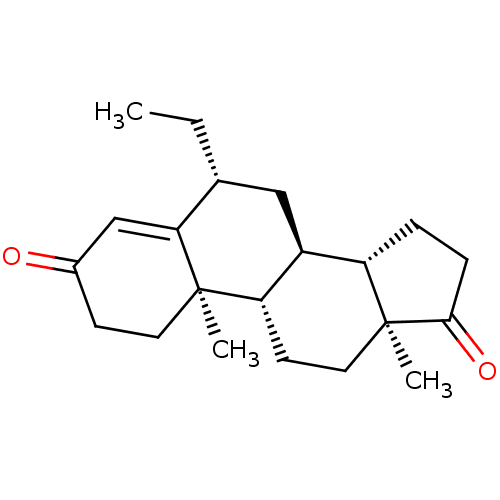 (CHEMBL3752661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136063 (CHEMBL3752102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135998 (CHEMBL3752668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135997 (CHEMBL3754471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136000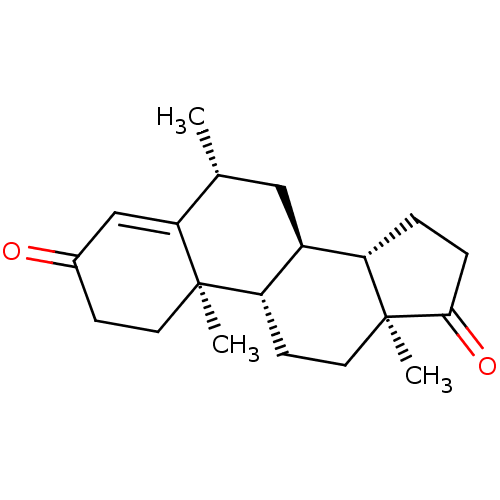 (CHEMBL3754220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393502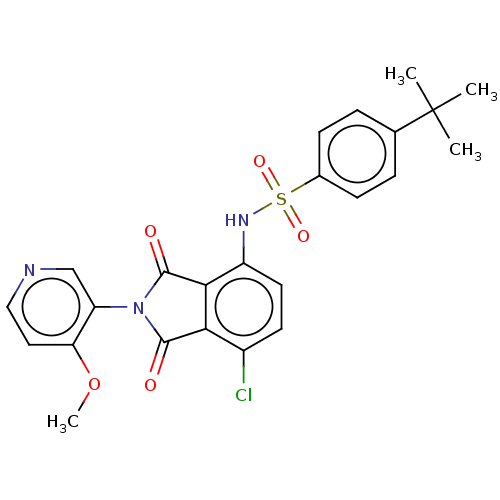 (US9969687, Compound 119) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291312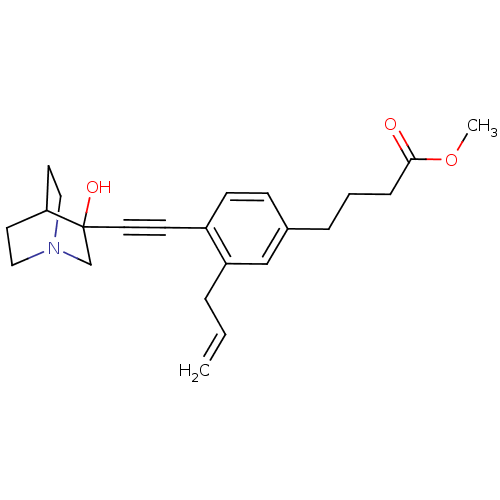 (4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264124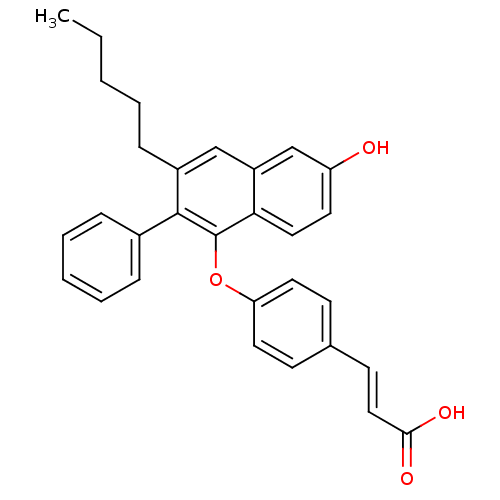 (3-(4-(6-hydroxy-3-pentyl-2-phenylnaphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135917 (CHEMBL3754546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135918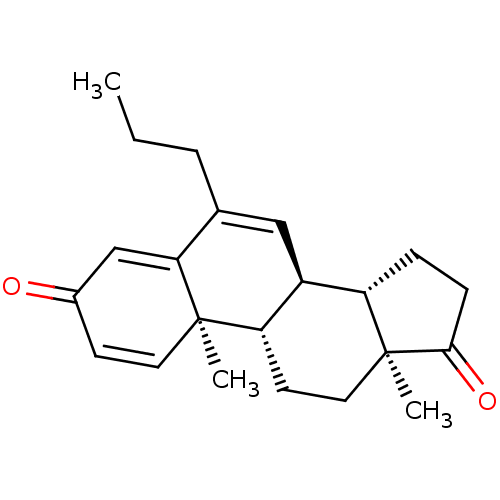 (CHEMBL3753803) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135921 (CHEMBL3752011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135922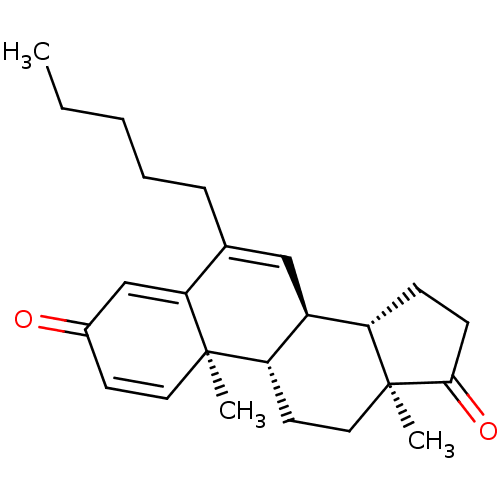 (CHEMBL3752619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135969 (CHEMBL3752315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135970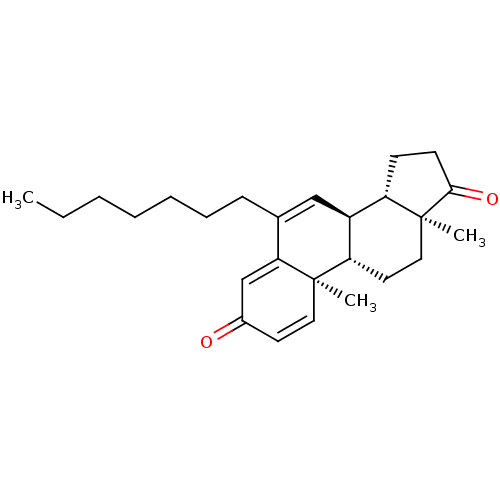 (CHEMBL3752341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135916 (CHEMBL3752165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50421878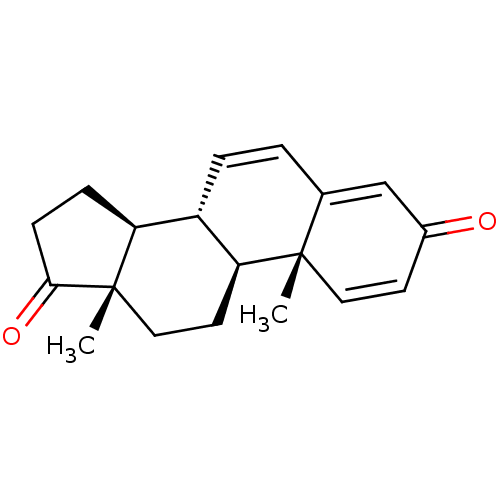 (CHEMBL2311178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135857 (CHEMBL3754285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135858 (CHEMBL3754366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135799 (CHEMBL3752650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393520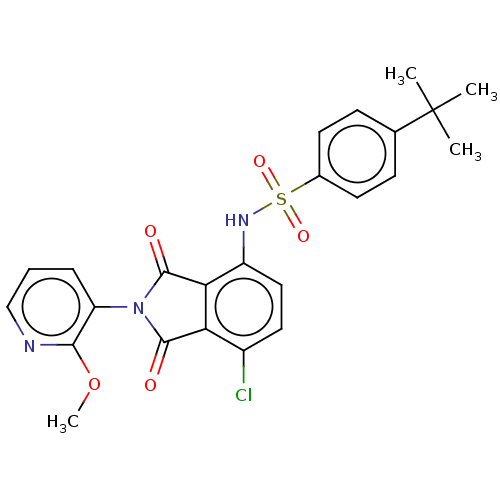 (US9969687, Compound 183) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9A receptor (unknown origin) overexpressed in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intrace... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264124 (3-(4-(6-hydroxy-3-pentyl-2-phenylnaphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393502 (US9969687, Compound 119) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCl25-mediated cell migration preincubated for 30 mins followed C... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393520 (US9969687, Compound 183) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393561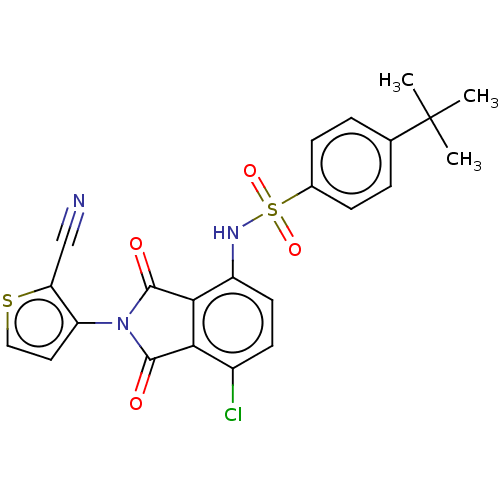 (US9969687, Compound 232) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description A calcium flux assay was used to determine the ability of the compounds to interfere with the binding between CCR9 and its chemokine ligand (TECK) in... | J Med Chem 52: 2652-5 (2009) BindingDB Entry DOI: 10.7270/Q28W3GNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393520 (US9969687, Compound 183) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description A calcium flux assay was used to determine the ability of the compounds to interfere with the binding between CCR9 and its chemokine ligand (TECK) in... | J Med Chem 52: 2652-5 (2009) BindingDB Entry DOI: 10.7270/Q28W3GNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393516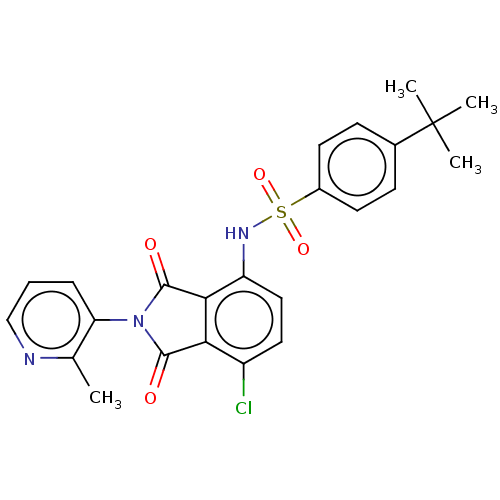 (US9969687, Compound 175) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393572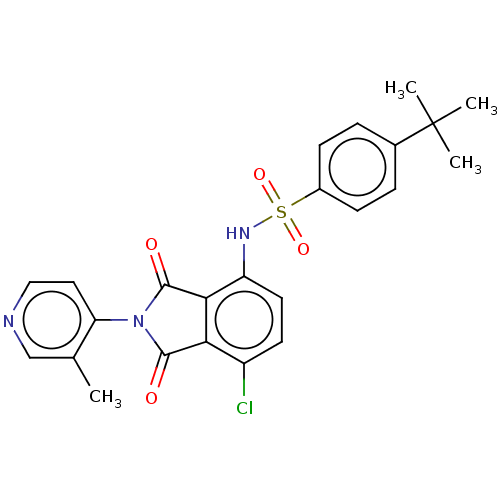 (US9969687, Compound 244) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description A calcium flux assay was used to determine the ability of the compounds to interfere with the binding between CCR9 and its chemokine ligand (TECK) in... | J Med Chem 52: 2652-5 (2009) BindingDB Entry DOI: 10.7270/Q28W3GNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135862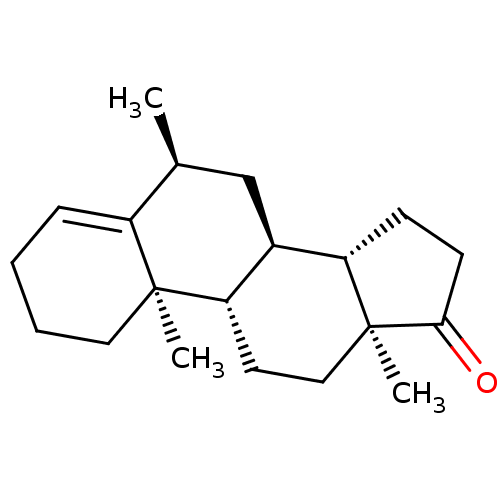 (CHEMBL3751881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332824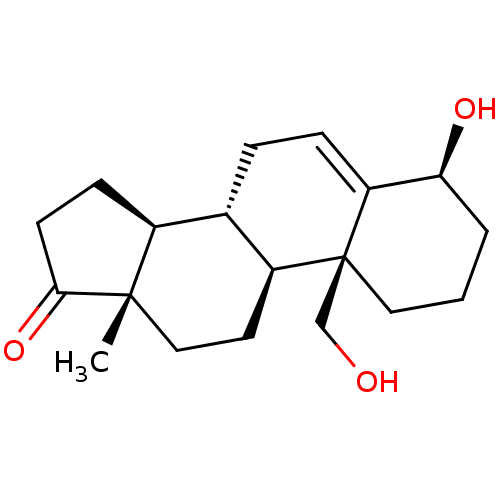 ((4S,8R,9S,10S,13S,14S)-4-hydroxy-10-(hydroxymethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136073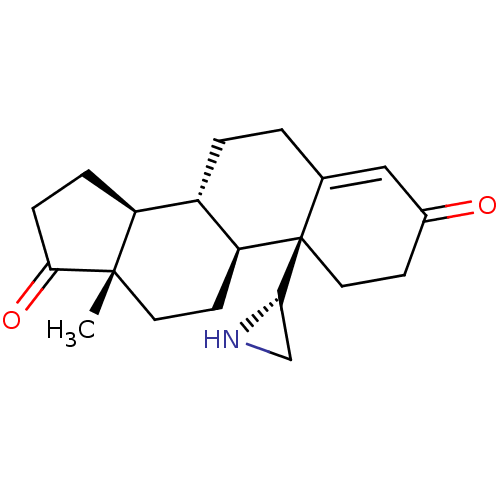 (CHEMBL3752276) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136076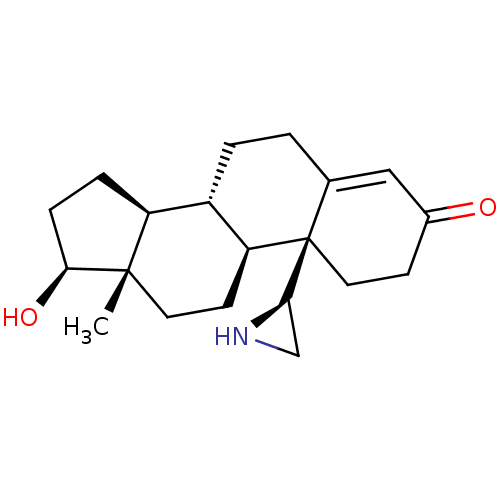 (CHEMBL3752403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136075 (CHEMBL3754519) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136074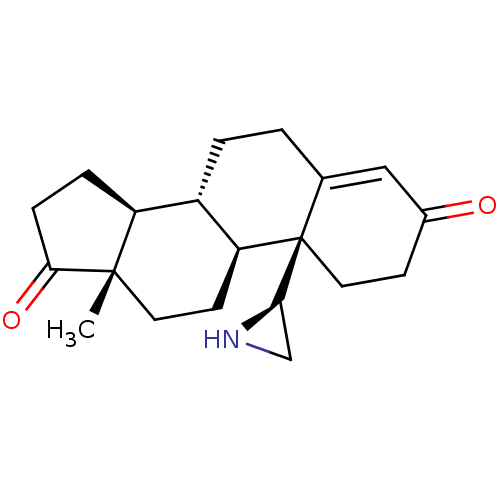 (CHEMBL3752897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM50398334 (CHEMBL2178578) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9A receptor (unknown origin) overexpressed in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intrace... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393600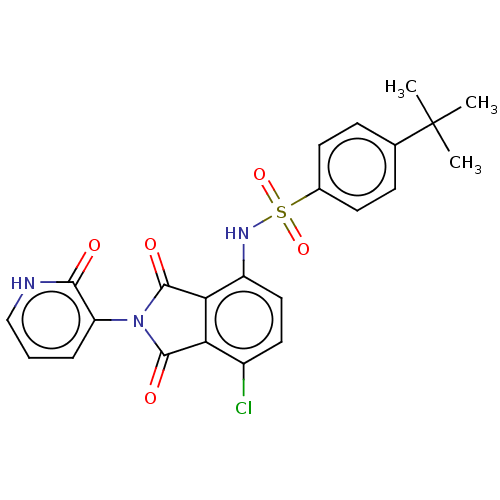 (US9969687, Compound 281) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description A calcium flux assay was used to determine the ability of the compounds to interfere with the binding between CCR9 and its chemokine ligand (TECK) in... | J Med Chem 52: 2652-5 (2009) BindingDB Entry DOI: 10.7270/Q28W3GNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393530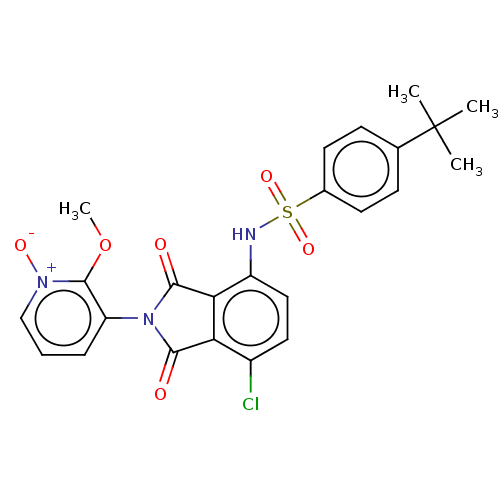 (US9969687, Compound 194) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264122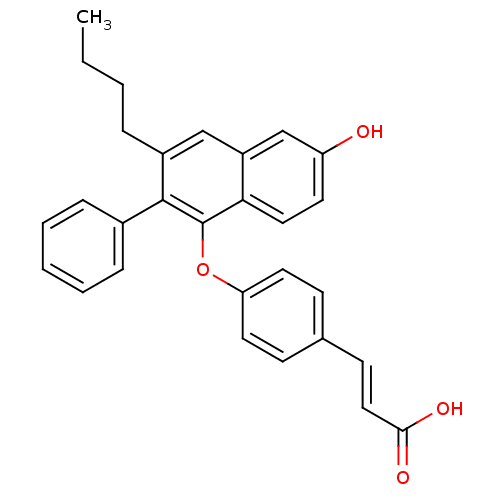 (3-(4-(3-butyl-6-hydroxy-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264122 (3-(4-(3-butyl-6-hydroxy-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136065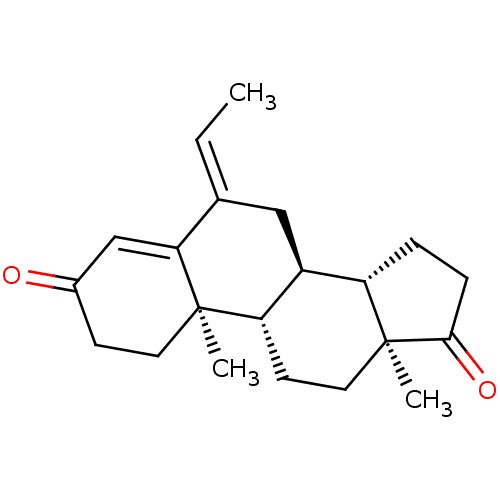 (CHEMBL3753629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393557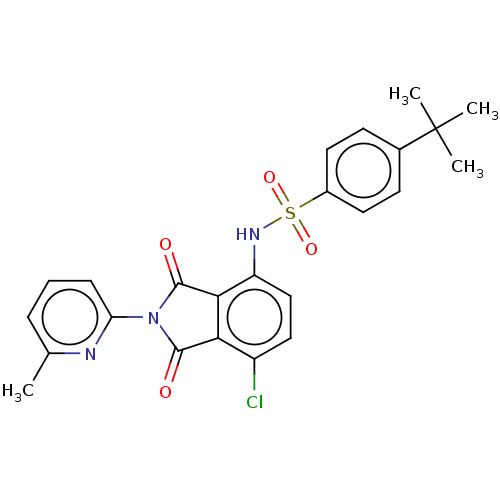 (US9969687, Compound 228) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393553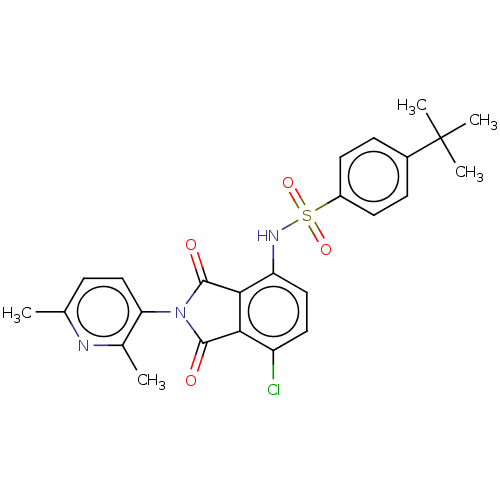 (US9969687, Compound 224) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50136064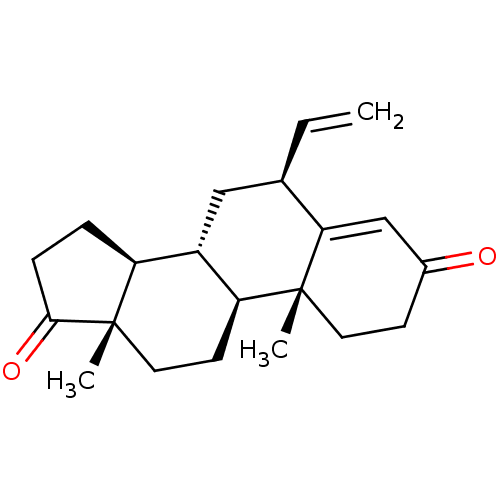 (CHEMBL3753920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135863 (CHEMBL3754711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD | Eur J Med Chem 105: 1-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.038 BindingDB Entry DOI: 10.7270/Q2W66NMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393530 (US9969687, Compound 194) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9A receptor (unknown origin) overexpressed in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intrace... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393552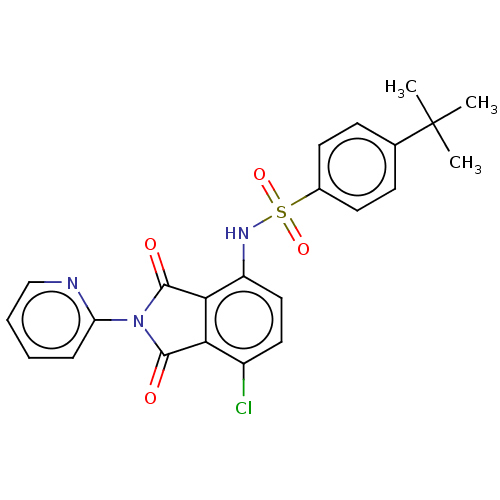 (US9969687, Compound 222) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1846 total ) | Next | Last >> |