 Found 3700 hits with Last Name = 'lang' and Initial = 's'
Found 3700 hits with Last Name = 'lang' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007377
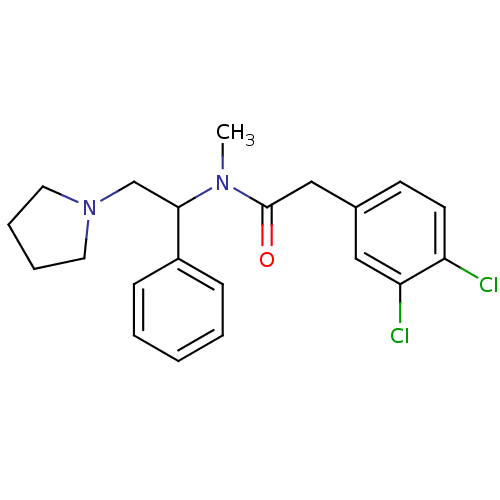
(2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2-pyr...)Show SMILES CN(C(CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for human Kappa opioid receptor |
Bioorg Med Chem Lett 14: 5693-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.041
BindingDB Entry DOI: 10.7270/Q27D2TMP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
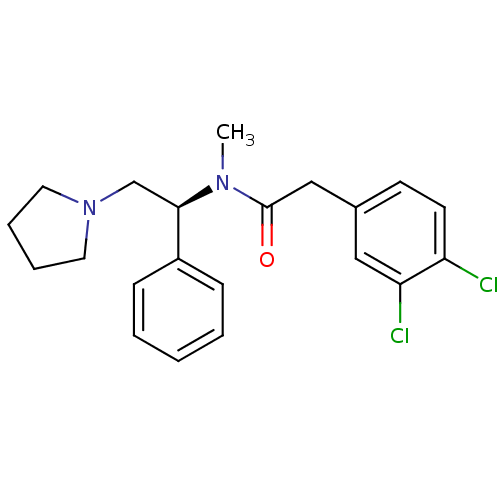
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176375
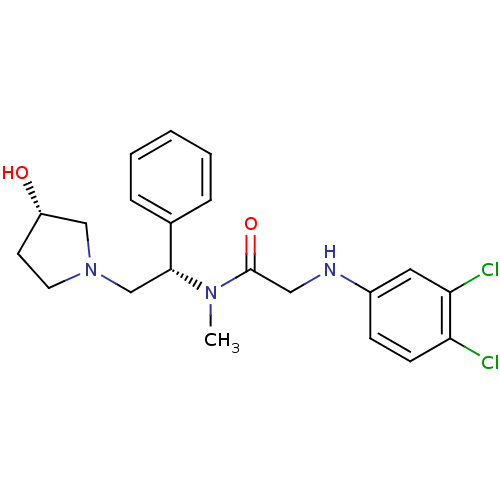
(2-(3,4-dichlorophenylamino)-N-((S)-2-((S)-3-hydrox...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H25Cl2N3O2/c1-25(21(28)12-24-16-7-8-18(22)19(23)11-16)20(15-5-3-2-4-6-15)14-26-10-9-17(27)13-26/h2-8,11,17,20,24,27H,9-10,12-14H2,1H3/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50517085

(CHEMBL4545711)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)s1 Show InChI InChI=1S/C10H7ClF3N5O3S2/c11-6-2-1-4(3-5(6)10(12,13)14)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h1-3H,(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176369
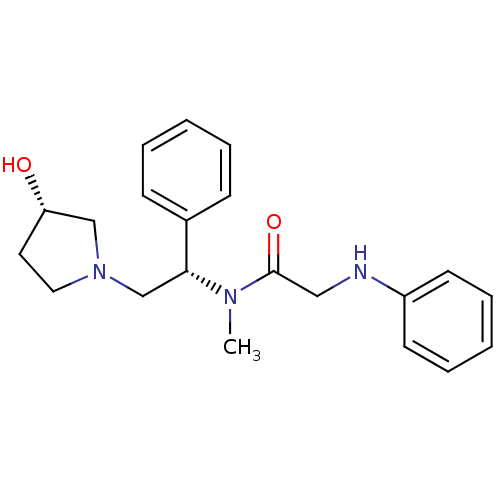
(CHEMBL201884 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1 Show InChI InChI=1S/C21H27N3O2/c1-23(21(26)14-22-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-24-13-12-19(25)15-24/h2-11,19-20,22,25H,12-16H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50155498

((S)-3-(3,4-Dichloro-phenyl)-1-[(S)-2-((S)-3-hydrox...)Show SMILES O[C@H]1CCN(C[C@@H](N2CC=CC[C@@H](c3ccc(Cl)c(Cl)c3)C2=O)c2ccccc2)C1 |c:9| Show InChI InChI=1S/C24H26Cl2N2O2/c25-21-10-9-18(14-22(21)26)20-8-4-5-12-28(24(20)30)23(17-6-2-1-3-7-17)16-27-13-11-19(29)15-27/h1-7,9-10,14,19-20,23,29H,8,11-13,15-16H2/t19-,20-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for human Kappa opioid receptor |
Bioorg Med Chem Lett 14: 5693-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.041
BindingDB Entry DOI: 10.7270/Q27D2TMP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176370
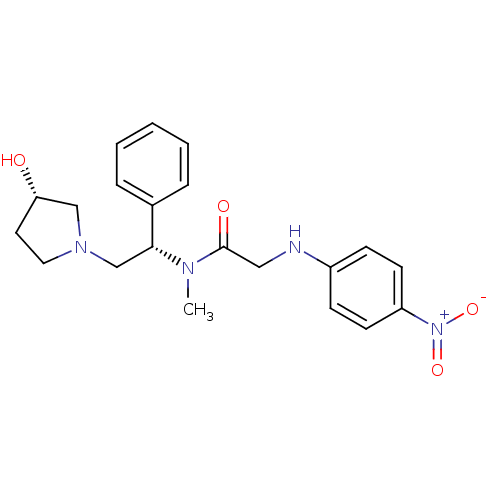
(CHEMBL201572 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-23(21(27)13-22-17-7-9-18(10-8-17)25(28)29)20(16-5-3-2-4-6-16)15-24-12-11-19(26)14-24/h2-10,19-20,22,26H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50517085

(CHEMBL4545711)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)s1 Show InChI InChI=1S/C10H7ClF3N5O3S2/c11-6-2-1-4(3-5(6)10(12,13)14)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h1-3H,(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50205684
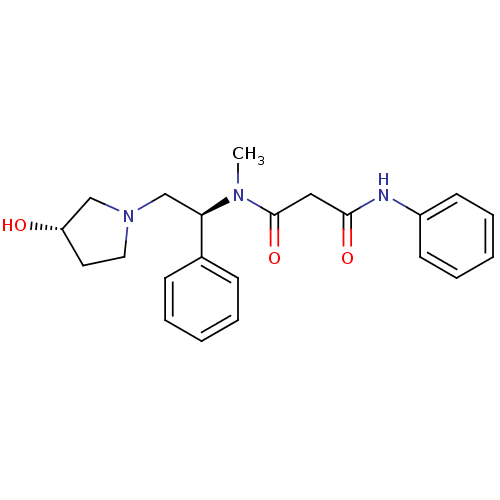
(CHEMBL230288 | N1-((S)-2-((S)-3-hydroxypyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CC(=O)Nc1ccccc1 Show InChI InChI=1S/C22H27N3O3/c1-24(22(28)14-21(27)23-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-25-13-12-19(26)15-25/h2-11,19-20,26H,12-16H2,1H3,(H,23,27)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 1951-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.053
BindingDB Entry DOI: 10.7270/Q2Q52P9X |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039026
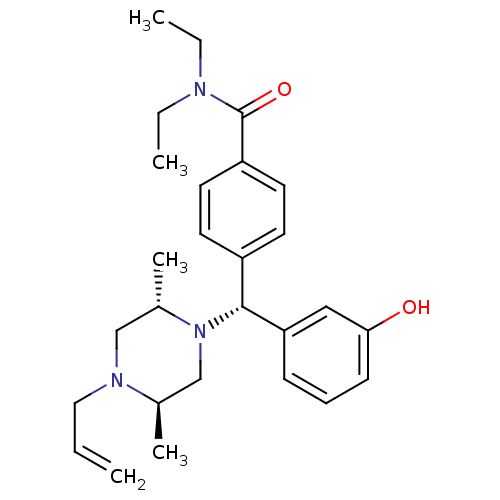
(4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(O)c1 Show InChI InChI=1S/C27H37N3O2/c1-6-16-29-18-21(5)30(19-20(29)4)26(24-10-9-11-25(31)17-24)22-12-14-23(15-13-22)27(32)28(7-2)8-3/h6,9-15,17,20-21,26,31H,1,7-8,16,18-19H2,2-5H3/t20-,21+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176374

(CHEMBL382932 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H29N3O2/c1-23(19-11-7-4-8-12-19)17-22(27)24(2)21(18-9-5-3-6-10-18)16-25-14-13-20(26)15-25/h3-12,20-21,26H,13-17H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50155494
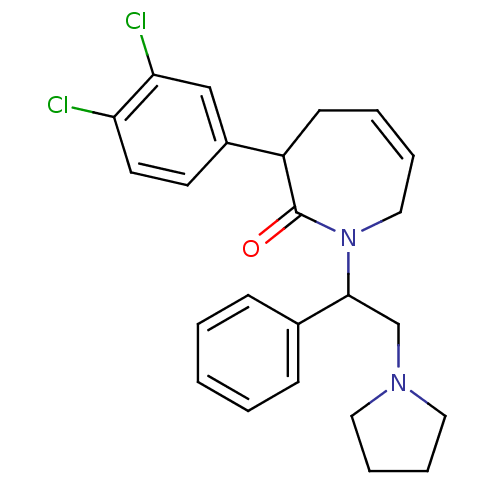
(3-(3,4-Dichloro-phenyl)-1-(1-phenyl-2-pyrrolidin-1...)Show SMILES Clc1ccc(cc1Cl)C1CC=CCN(C(CN2CCCC2)c2ccccc2)C1=O |c:11| Show InChI InChI=1S/C24H26Cl2N2O/c25-21-12-11-19(16-22(21)26)20-10-4-5-15-28(24(20)29)23(17-27-13-6-7-14-27)18-8-2-1-3-9-18/h1-5,8-9,11-12,16,20,23H,6-7,10,13-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for human Kappa opioid receptor |
Bioorg Med Chem Lett 14: 5693-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.041
BindingDB Entry DOI: 10.7270/Q27D2TMP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50517085

(CHEMBL4545711)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)s1 Show InChI InChI=1S/C10H7ClF3N5O3S2/c11-6-2-1-4(3-5(6)10(12,13)14)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h1-3H,(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176373
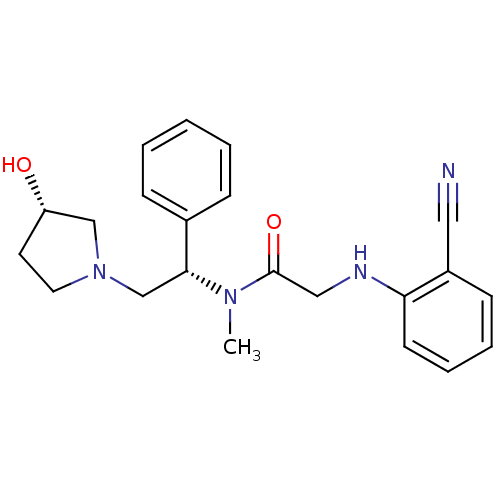
(2-(2-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-20-10-6-5-9-18(20)13-23)21(17-7-3-2-4-8-17)16-26-12-11-19(27)15-26/h2-10,19,21,24,27H,11-12,14-16H2,1H3/t19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176365
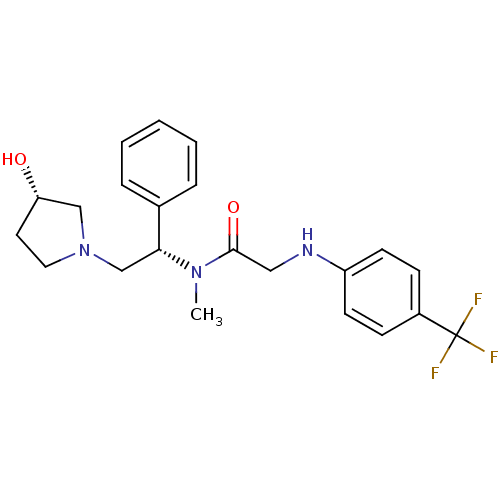
(CHEMBL201905 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c1-27(21(30)13-26-18-9-7-17(8-10-18)22(23,24)25)20(16-5-3-2-4-6-16)15-28-12-11-19(29)14-28/h2-10,19-20,26,29H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166621
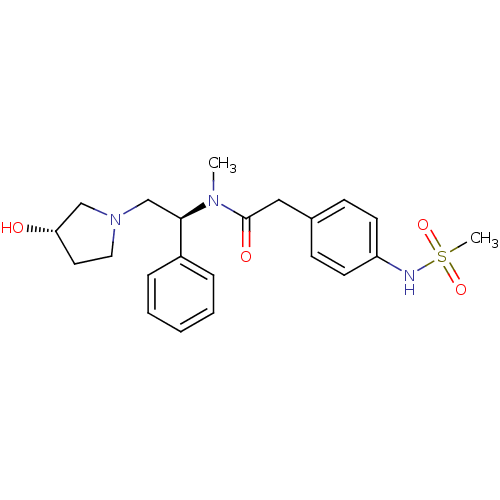
(CHEMBL191987 | N-[(S)-2-((S)-3-Hydroxy-pyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)Cc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H29N3O4S/c1-24(22(27)14-17-8-10-19(11-9-17)23-30(2,28)29)21(18-6-4-3-5-7-18)16-25-13-12-20(26)15-25/h3-11,20-21,23,26H,12-16H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86965
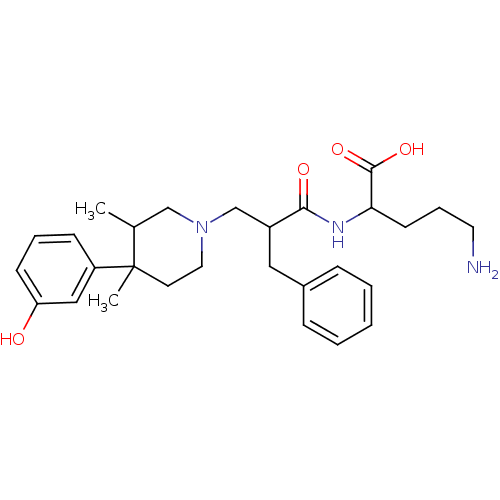
((S)-5-amino-2-((S)-2-(((3R,4R)-4-(3-hydroxyphenyl)...)Show SMILES CC1CN(CC(Cc2ccccc2)C(=O)NC(CCCN)C(O)=O)CCC1(C)c1cccc(O)c1 Show InChI InChI=1S/C28H39N3O4/c1-20-18-31(15-13-28(20,2)23-10-6-11-24(32)17-23)19-22(16-21-8-4-3-5-9-21)26(33)30-25(27(34)35)12-7-14-29/h3-6,8-11,17,20,22,25,32H,7,12-16,18-19,29H2,1-2H3,(H,30,33)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 2006-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.106
BindingDB Entry DOI: 10.7270/Q2B856Q8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50252953
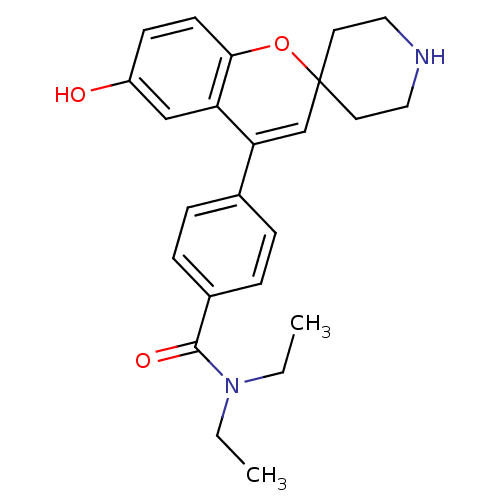
(CHEMBL494479 | N,N-Diethyl-4-(6-hydroxyspiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccc(O)cc12 |t:14| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)18-7-5-17(6-8-18)21-16-24(11-13-25-14-12-24)29-22-10-9-19(27)15-20(21)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199910
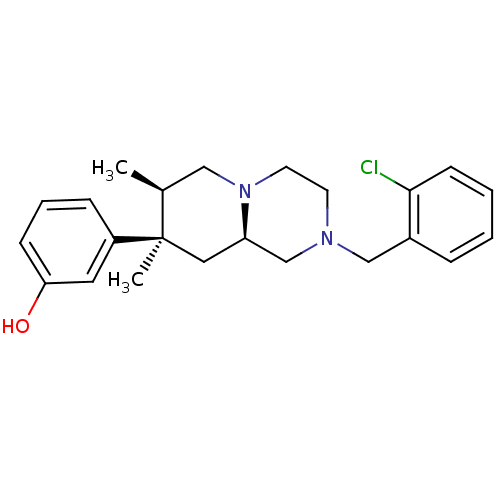
(3-((7R,8R,9alphaR)-7,8-dimethyl-2-(2-chlorobenzyl)...)Show SMILES C[C@H]1CN2CCN(Cc3ccccc3Cl)C[C@H]2C[C@@]1(C)c1cccc(O)c1 |r| Show InChI InChI=1S/C23H29ClN2O/c1-17-14-26-11-10-25(15-18-6-3-4-9-22(18)24)16-20(26)13-23(17,2)19-7-5-8-21(27)12-19/h3-9,12,17,20,27H,10-11,13-16H2,1-2H3/t17-,20+,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells |
J Med Chem 49: 7290-306 (2006)
Article DOI: 10.1021/jm0604878
BindingDB Entry DOI: 10.7270/Q2CF9QW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86964
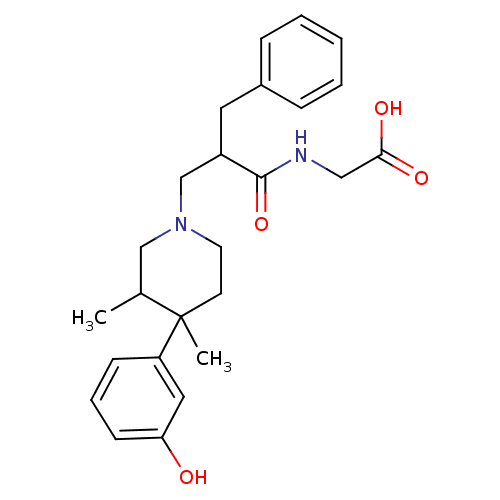
(2-((S)-2-benzyl-3-((3R,4R)-4-(3-hydroxyphenyl)-3,4...)Show SMILES CC1CN(CC(Cc2ccccc2)C(=O)NCC(O)=O)CCC1(C)c1cccc(O)c1 Show InChI InChI=1S/C25H32N2O4/c1-18-16-27(12-11-25(18,2)21-9-6-10-22(28)14-21)17-20(24(31)26-15-23(29)30)13-19-7-4-3-5-8-19/h3-10,14,18,20,28H,11-13,15-17H2,1-2H3,(H,26,31)(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 2006-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.106
BindingDB Entry DOI: 10.7270/Q2B856Q8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50136591
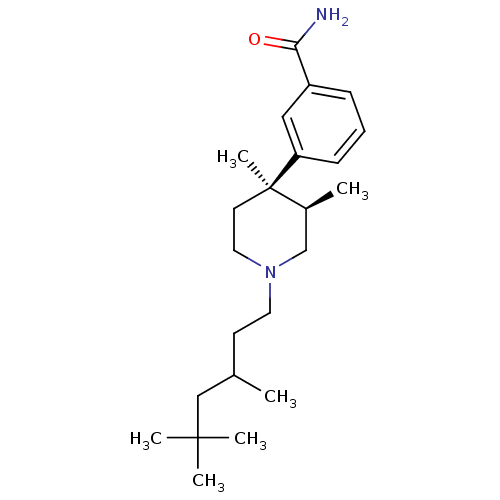
(3-[(3R,4R)-3,4-Dimethyl-1-(3,5,5-trimethyl-hexyl)-...)Show SMILES CC(CCN1CC[C@](C)([C@@H](C)C1)c1cccc(c1)C(N)=O)CC(C)(C)C Show InChI InChI=1S/C23H38N2O/c1-17(15-22(3,4)5)10-12-25-13-11-23(6,18(2)16-25)20-9-7-8-19(14-20)21(24)26/h7-9,14,17-18H,10-13,15-16H2,1-6H3,(H2,24,26)/t17?,18-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding of the non-selective opioid antagonist, [3H]diprenorphine, to cloned human mu opioid receptor |
Bioorg Med Chem Lett 13: 4459-62 (2003)
BindingDB Entry DOI: 10.7270/Q2RV0P7G |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176367
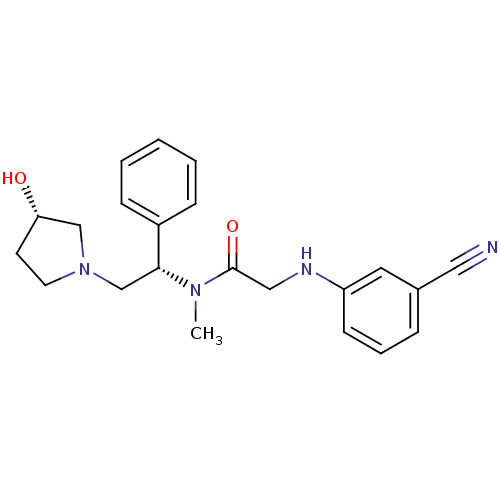
(2-(3-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(c1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-5-6-17(12-19)13-23)21(18-7-3-2-4-8-18)16-26-11-10-20(27)15-26/h2-9,12,20-21,24,27H,10-11,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86968
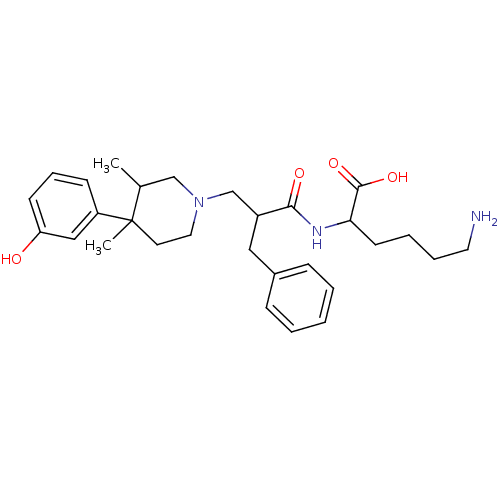
((S)-6-amino-2-((S)-2-(((3R,4R)-4-(3-hydroxyphenyl)...)Show SMILES CC1CN(CC(Cc2ccccc2)C(=O)NC(CCCCN)C(O)=O)CCC1(C)c1cccc(O)c1 Show InChI InChI=1S/C29H41N3O4/c1-21-19-32(16-14-29(21,2)24-11-8-12-25(33)18-24)20-23(17-22-9-4-3-5-10-22)27(34)31-26(28(35)36)13-6-7-15-30/h3-5,8-12,18,21,23,26,33H,6-7,13-17,19-20,30H2,1-2H3,(H,31,34)(H,35,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 2006-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.106
BindingDB Entry DOI: 10.7270/Q2B856Q8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50205686
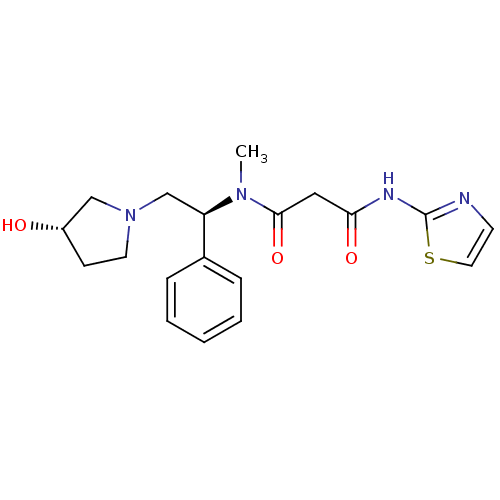
(CHEMBL230607 | N1-((S)-2-((S)-3-hydroxypyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CC(=O)Nc1nccs1 Show InChI InChI=1S/C19H24N4O3S/c1-22(18(26)11-17(25)21-19-20-8-10-27-19)16(14-5-3-2-4-6-14)13-23-9-7-15(24)12-23/h2-6,8,10,15-16,24H,7,9,11-13H2,1H3,(H,20,21,25)/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 1951-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.053
BindingDB Entry DOI: 10.7270/Q2Q52P9X |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297321

(CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1Cc2ccccc2C1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:20| Show InChI InChI=1S/C28H26N2O2/c31-27(30-18-22-5-1-2-6-23(22)19-30)21-11-9-20(10-12-21)25-17-28(13-15-29-16-14-28)32-26-8-4-3-7-24(25)26/h1-12,17,29H,13-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166619
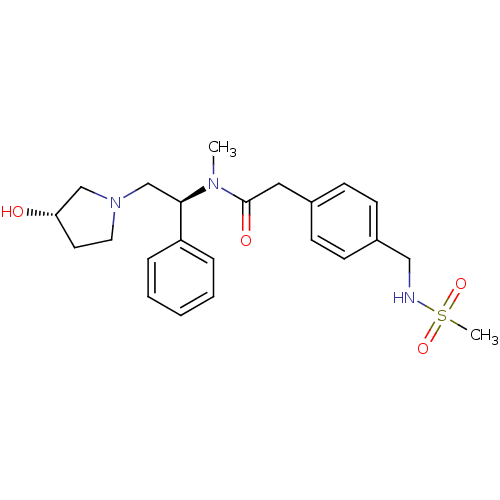
(CHEMBL425897 | N-[(S)-2-((S)-3-Hydroxy-pyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)Cc1ccc(CNS(C)(=O)=O)cc1 Show InChI InChI=1S/C23H31N3O4S/c1-25(22(20-6-4-3-5-7-20)17-26-13-12-21(27)16-26)23(28)14-18-8-10-19(11-9-18)15-24-31(2,29)30/h3-11,21-22,24,27H,12-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199896
(3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...)Show SMILES C[C@H]1CN2C[C@@H](CC[C@H]2C[C@@]1(C)c1cccc(O)c1)c1ccccc1 |r| Show InChI InChI=1S/C23H29NO/c1-17-15-24-16-19(18-7-4-3-5-8-18)11-12-21(24)14-23(17,2)20-9-6-10-22(25)13-20/h3-10,13,17,19,21,25H,11-12,14-16H2,1-2H3/t17-,19+,21-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells |
J Med Chem 49: 7290-306 (2006)
Article DOI: 10.1021/jm0604878
BindingDB Entry DOI: 10.7270/Q2CF9QW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199896

(3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...)Show SMILES C[C@H]1CN2C[C@@H](CC[C@H]2C[C@@]1(C)c1cccc(O)c1)c1ccccc1 |r| Show InChI InChI=1S/C23H29NO/c1-17-15-24-16-19(18-7-4-3-5-8-18)11-12-21(24)14-23(17,2)20-9-6-10-22(25)13-20/h3-10,13,17,19,21,25H,11-12,14-16H2,1-2H3/t17-,19+,21-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells |
J Med Chem 49: 7278-89 (2006)
Article DOI: 10.1021/jm060486f
BindingDB Entry DOI: 10.7270/Q27P906S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86969
((R)-2-((S)-2-(((3R,4R)-4-(3-hydroxyphenyl)-3,4-dim...)Show SMILES CC(NC(=O)C(CN1CCC(C)(C(C)C1)c1cccc(O)c1)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C26H34N2O4/c1-18-16-28(13-12-26(18,3)22-10-7-11-23(29)15-22)17-21(14-20-8-5-4-6-9-20)24(30)27-19(2)25(31)32/h4-11,15,18-19,21,29H,12-14,16-17H2,1-3H3,(H,27,30)(H,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 2006-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.106
BindingDB Entry DOI: 10.7270/Q2B856Q8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50517085

(CHEMBL4545711)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)s1 Show InChI InChI=1S/C10H7ClF3N5O3S2/c11-6-2-1-4(3-5(6)10(12,13)14)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h1-3H,(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA1 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176376
(2-(4-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-7-17(13-23)8-10-19)21(18-5-3-2-4-6-18)16-26-12-11-20(27)15-26/h2-10,20-21,24,27H,11-12,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297339
(CHEMBL557458 | N,N-Diethyl-2-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(cc1O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)19-10-9-17(15-21(19)27)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50517082
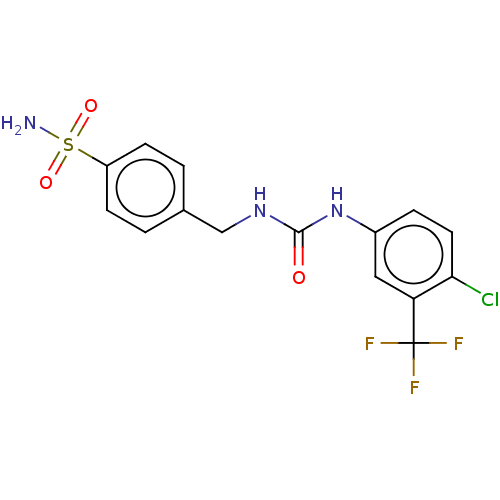
(CHEMBL4466537)Show SMILES NS(=O)(=O)c1ccc(CNC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C15H13ClF3N3O3S/c16-13-6-3-10(7-12(13)15(17,18)19)22-14(23)21-8-9-1-4-11(5-2-9)26(20,24)25/h1-7H,8H2,(H2,20,24,25)(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50155495
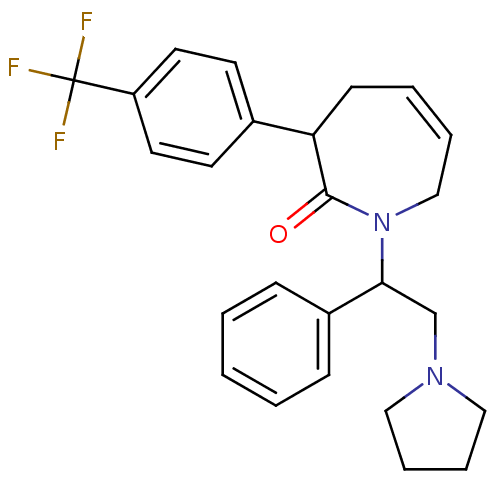
(1-(1-Phenyl-2-pyrrolidin-1-yl-ethyl)-3-(4-trifluor...)Show SMILES FC(F)(F)c1ccc(cc1)C1CC=CCN(C(CN2CCCC2)c2ccccc2)C1=O |c:13| Show InChI InChI=1S/C25H27F3N2O/c26-25(27,28)21-13-11-19(12-14-21)22-10-4-5-17-30(24(22)31)23(18-29-15-6-7-16-29)20-8-2-1-3-9-20/h1-5,8-9,11-14,22-23H,6-7,10,15-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for human Kappa opioid receptor |
Bioorg Med Chem Lett 14: 5693-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.041
BindingDB Entry DOI: 10.7270/Q27D2TMP |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50207832
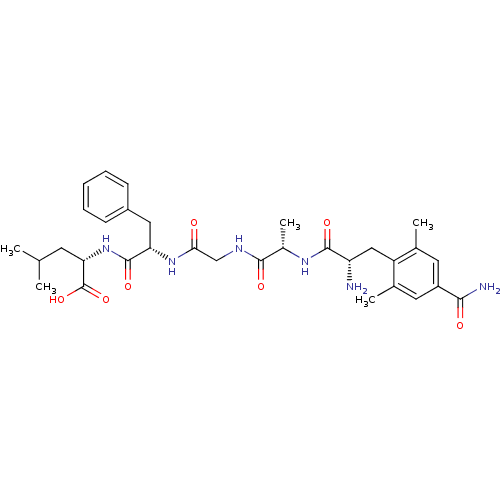
(CHEMBL247758 | H-Cdp-ala-Gly-Phe-leu-OH)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(cc1C)C(N)=O)C(O)=O Show InChI InChI=1S/C32H44N6O7/c1-17(2)11-26(32(44)45)38-31(43)25(14-21-9-7-6-8-10-21)37-27(39)16-35-29(41)20(5)36-30(42)24(33)15-23-18(3)12-22(28(34)40)13-19(23)4/h6-10,12-13,17,20,24-26H,11,14-16,33H2,1-5H3,(H2,34,40)(H,35,41)(H,36,42)(H,37,39)(H,38,43)(H,44,45)/t20-,24-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned delta opioid receptor |
Bioorg Med Chem Lett 17: 2656-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.092
BindingDB Entry DOI: 10.7270/Q27S7NGH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176372
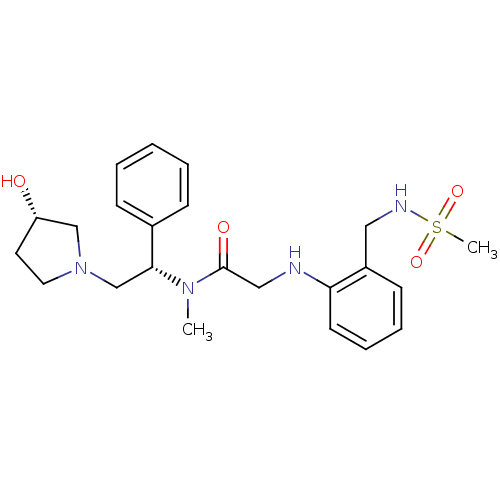
(CHEMBL201283 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1CNS(C)(=O)=O Show InChI InChI=1S/C23H32N4O4S/c1-26(22(18-8-4-3-5-9-18)17-27-13-12-20(28)16-27)23(29)15-24-21-11-7-6-10-19(21)14-25-32(2,30)31/h3-11,20,22,24-25,28H,12-17H2,1-2H3/t20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174961
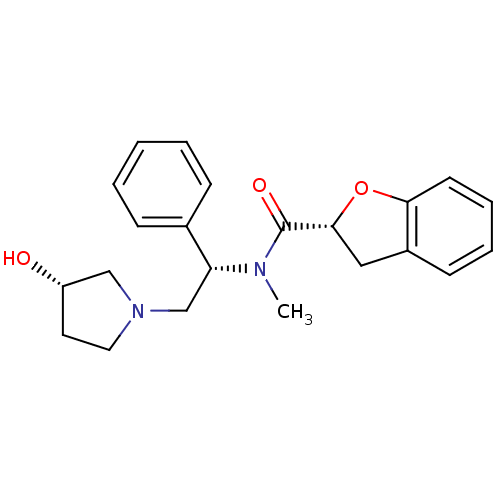
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1Cc2ccccc2O1 Show InChI InChI=1S/C22H26N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,18-19,21,25H,11-15H2,1H3/t18-,19+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166613
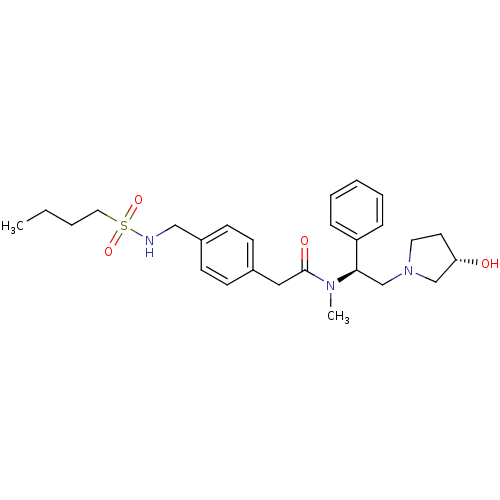
(2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[...)Show SMILES CCCCS(=O)(=O)NCc1ccc(CC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C26H37N3O4S/c1-3-4-16-34(32,33)27-18-22-12-10-21(11-13-22)17-26(31)28(2)25(23-8-6-5-7-9-23)20-29-15-14-24(30)19-29/h5-13,24-25,27,30H,3-4,14-20H2,1-2H3/t24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50517088
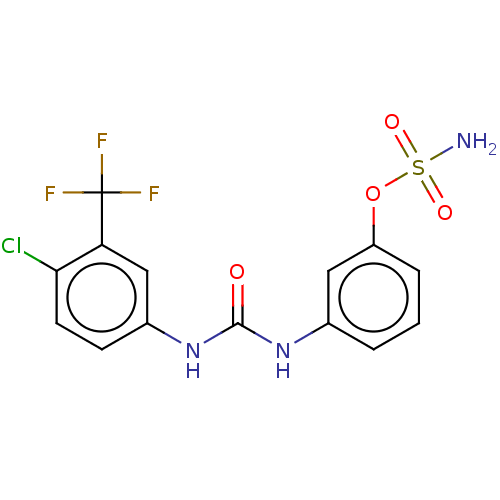
(CHEMBL4543156)Show SMILES NS(=O)(=O)Oc1cccc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c1 Show InChI InChI=1S/C14H11ClF3N3O4S/c15-12-5-4-9(7-11(12)14(16,17)18)21-13(22)20-8-2-1-3-10(6-8)25-26(19,23)24/h1-7H,(H2,19,23,24)(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50252954
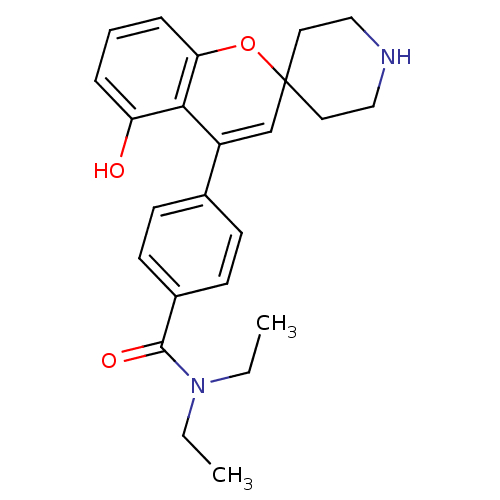
(ADL-5859 | CHEMBL494480 | N,N-diethyl-4-(5-hydroxy...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2cccc(O)c12 |t:14| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)18-10-8-17(9-11-18)19-16-24(12-14-25-15-13-24)29-21-7-5-6-20(27)22(19)21/h5-11,16,25,27H,3-4,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199900
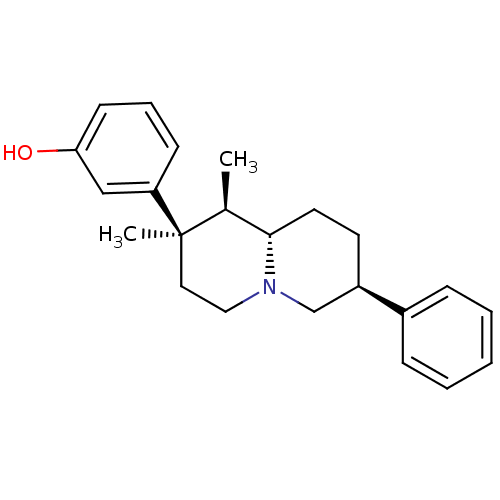
(3-((1R,2R,7S,9-alpha-S)-1,2-dimethyl-7-phenyl-octa...)Show SMILES C[C@H]1[C@@H]2CC[C@H](CN2CC[C@@]1(C)c1cccc(O)c1)c1ccccc1 |r| Show InChI InChI=1S/C23H29NO/c1-17-22-12-11-19(18-7-4-3-5-8-18)16-24(22)14-13-23(17,2)20-9-6-10-21(25)15-20/h3-10,15,17,19,22,25H,11-14,16H2,1-2H3/t17-,19+,22-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against human cloned mu opioid receptor expressed in CHO cells assessed as inhibition of loperamide-stimulated [35S]GTP-gamma-S b... |
J Med Chem 49: 7278-89 (2006)
Article DOI: 10.1021/jm060486f
BindingDB Entry DOI: 10.7270/Q27P906S |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297329
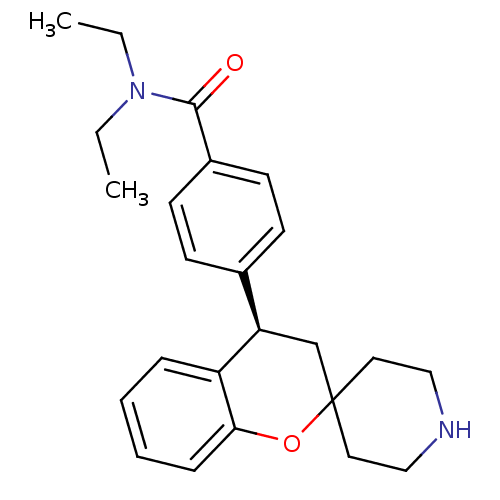
((R)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199945
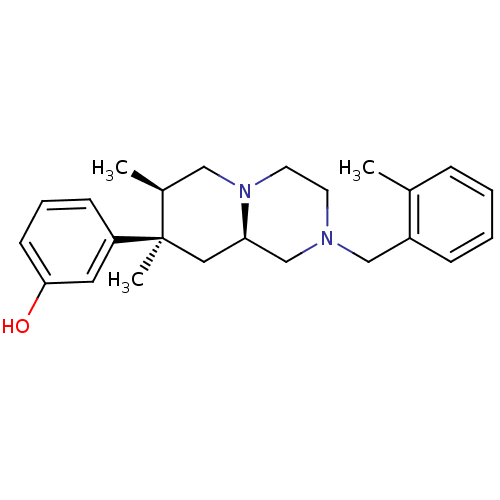
(3-((7R,8R,9alphaR)-7,8-dimethyl-2-(2-methylbenzyl)...)Show SMILES C[C@H]1CN2CCN(Cc3ccccc3C)C[C@H]2C[C@@]1(C)c1cccc(O)c1 |r| Show InChI InChI=1S/C24H32N2O/c1-18-7-4-5-8-20(18)16-25-11-12-26-15-19(2)24(3,14-22(26)17-25)21-9-6-10-23(27)13-21/h4-10,13,19,22,27H,11-12,14-17H2,1-3H3/t19-,22+,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells |
J Med Chem 49: 7290-306 (2006)
Article DOI: 10.1021/jm0604878
BindingDB Entry DOI: 10.7270/Q2CF9QW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells |
J Med Chem 49: 7290-306 (2006)
Article DOI: 10.1021/jm0604878
BindingDB Entry DOI: 10.7270/Q2CF9QW8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176362

(2-((4-cyanophenyl)(methyl)amino)-N-((S)-2-((S)-3-h...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H28N4O2/c1-25(20-10-8-18(14-24)9-11-20)17-23(29)26(2)22(19-6-4-3-5-7-19)16-27-13-12-21(28)15-27/h3-11,21-22,28H,12-13,15-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells |
J Med Chem 49: 7278-89 (2006)
Article DOI: 10.1021/jm060486f
BindingDB Entry DOI: 10.7270/Q27P906S |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50207833
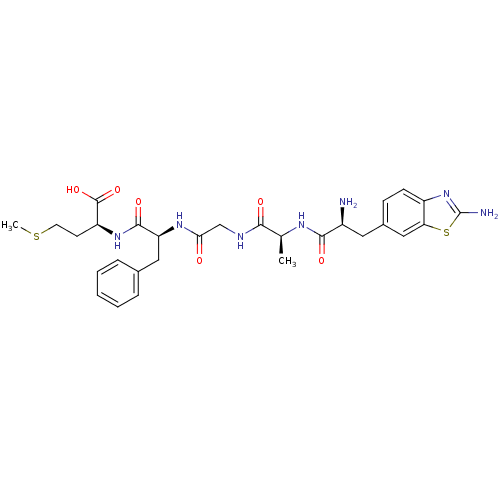
(CHEMBL246572 | H-Aba-ala-Gly-Phe-Met-OH)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc2nc(N)sc2c1)C(O)=O Show InChI InChI=1S/C29H37N7O6S2/c1-16(33-26(39)19(30)12-18-8-9-20-23(14-18)44-29(31)36-20)25(38)32-15-24(37)34-22(13-17-6-4-3-5-7-17)27(40)35-21(28(41)42)10-11-43-2/h3-9,14,16,19,21-22H,10-13,15,30H2,1-2H3,(H2,31,36)(H,32,38)(H,33,39)(H,34,37)(H,35,40)(H,41,42)/t16-,19-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned delta opioid receptor |
Bioorg Med Chem Lett 17: 2656-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.092
BindingDB Entry DOI: 10.7270/Q27S7NGH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50387125
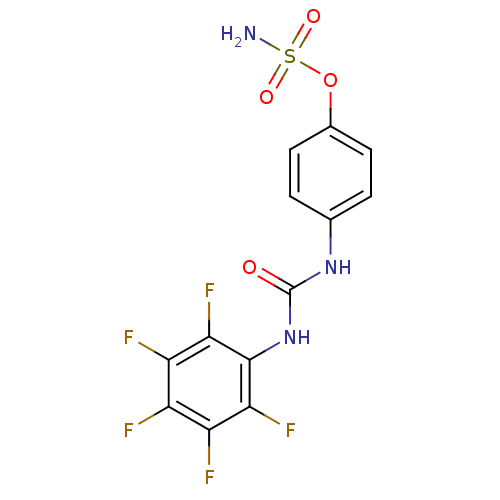
(4-ureidophenyl sulfamate ring derivative 3j | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C13H8F5N3O4S/c14-7-8(15)10(17)12(11(18)9(7)16)21-13(22)20-5-1-3-6(4-2-5)25-26(19,23)24/h1-4H,(H2,19,23,24)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay |
Bioorg Med Chem Lett 22: 4681-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.083
BindingDB Entry DOI: 10.7270/Q20866C2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50207825
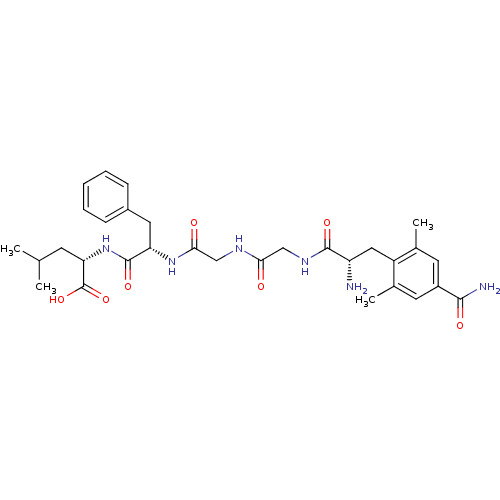
(CHEMBL246739 | H-Cdp-Gly-Gly-Phe-Leu-OH)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1c(C)cc(cc1C)C(N)=O)C(O)=O Show InChI InChI=1S/C31H42N6O7/c1-17(2)10-25(31(43)44)37-30(42)24(13-20-8-6-5-7-9-20)36-27(39)16-34-26(38)15-35-29(41)23(32)14-22-18(3)11-21(28(33)40)12-19(22)4/h5-9,11-12,17,23-25H,10,13-16,32H2,1-4H3,(H2,33,40)(H,34,38)(H,35,41)(H,36,39)(H,37,42)(H,43,44)/t23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned delta opioid receptor |
Bioorg Med Chem Lett 17: 2656-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.092
BindingDB Entry DOI: 10.7270/Q27S7NGH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199941
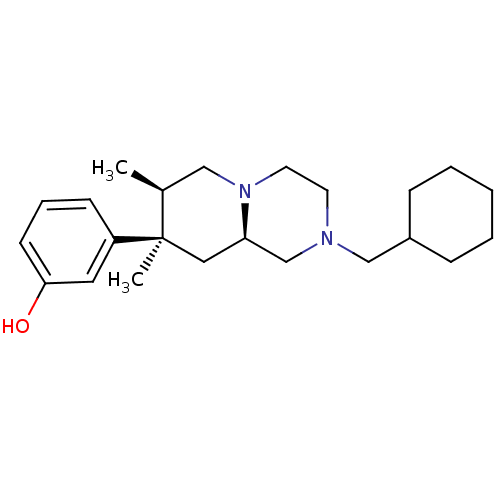
(3-((7R,8R,9alphaR)-2-(cyclohexylmethyl)-7,8-dimeth...)Show SMILES C[C@H]1CN2CCN(CC3CCCCC3)C[C@H]2C[C@@]1(C)c1cccc(O)c1 |r| Show InChI InChI=1S/C23H36N2O/c1-18-15-25-12-11-24(16-19-7-4-3-5-8-19)17-21(25)14-23(18,2)20-9-6-10-22(26)13-20/h6,9-10,13,18-19,21,26H,3-5,7-8,11-12,14-17H2,1-2H3/t18-,21+,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells |
J Med Chem 49: 7290-306 (2006)
Article DOI: 10.1021/jm0604878
BindingDB Entry DOI: 10.7270/Q2CF9QW8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data