| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Bifunctional epoxide hydrolase 2 |
|---|
| Ligand | BDBM50191858 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_393605 (CHEMBL912696) |
|---|
| IC50 | 2.7±n/a nM |
|---|
| Citation |  Jones, PD; Tsai, HJ; Do, ZN; Morisseau, C; Hammock, BD Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett16:5212-6 (2006) [PubMed] Article Jones, PD; Tsai, HJ; Do, ZN; Morisseau, C; Hammock, BD Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett16:5212-6 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Bifunctional epoxide hydrolase 2 |
|---|
| Name: | Bifunctional epoxide hydrolase 2 |
|---|
| Synonyms: | Cytosolic epoxide hydrolase 2 | EBifunctional epoxide hydrolase 2 | EPHX2 | Epoxide hydratase | HYES_HUMAN | Lipid-phosphate phosphatase | Soluble epoxide hydrolase (sEH) | epoxide hydrolase 2, cytoplasmic |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 62613.07 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P34913 |
|---|
| Residue: | 555 |
|---|
| Sequence: | MTLRAAVFDLDGVLALPAVFGVLGRTEEALALPRGLLNDAFQKGGPEGATTRLMKGEITL
SQWIPLMEENCRKCSETAKVCLPKNFSIKEIFDKAISARKINRPMLQAALMLRKKGFTTA
ILTNTWLDDRAERDGLAQLMCELKMHFDFLIESCQVGMVKPEPQIYKFLLDTLKASPSEV
VFLDDIGANLKPARDLGMVTILVQDTDTALKELEKVTGIQLLNTPAPLPTSCNPSDMSHG
YVTVKPRVRLHFVELGSGPAVCLCHGFPESWYSWRYQIPALAQAGYRVLAMDMKGYGESS
APPEIEEYCMEVLCKEMVTFLDKLGLSQAVFIGHDWGGMLVWYMALFYPERVRAVASLNT
PFIPANPNMSPLESIKANPVFDYQLYFQEPGVAEAELEQNLSRTFKSLFRASDESVLSMH
KVCEAGGLFVNSPEEPSLSRMVTEEEIQFYVQQFKKSGFRGPLNWYRNMERNWKWACKSL
GRKILIPALMVTAEKDFVLVPQMSQHMEDWIPHLKRGHIEDCGHWTQMDKPTEVNQILIK
WLDSDARNPPVVSKM
|
|
|
|---|
| BDBM50191858 |
|---|
| n/a |
|---|
| Name | BDBM50191858 |
|---|
| Synonyms: | 5-[4-(3-adamantan-1-yl-ureido)-piperidin-1-yl]-5-oxo-pentanoic acid methyl ester | CHEMBL214505 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H35N3O4 |
|---|
| Mol. Mass. | 405.531 |
|---|
| SMILES | COC(=O)CCCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24| |
|---|
| Structure | 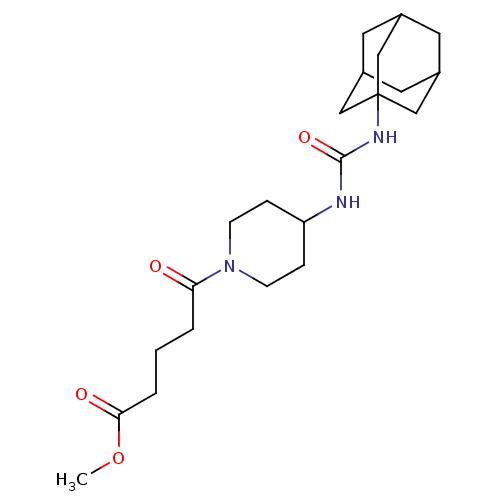
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jones, PD; Tsai, HJ; Do, ZN; Morisseau, C; Hammock, BD Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett16:5212-6 (2006) [PubMed] Article
Jones, PD; Tsai, HJ; Do, ZN; Morisseau, C; Hammock, BD Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett16:5212-6 (2006) [PubMed] Article