

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50135653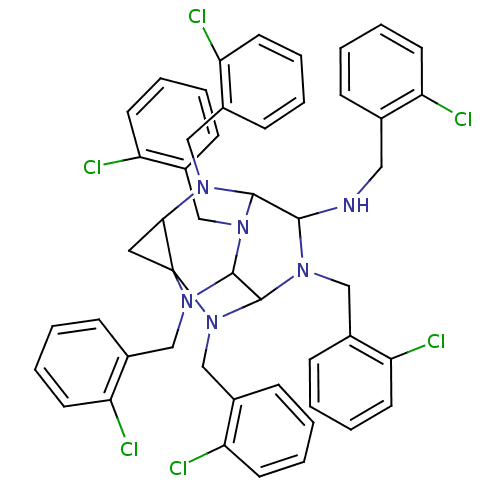 (2,4,6,8,10,12-hexa(2-chloro phenylmethyl)-2,4,6,8,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Butyrylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50192958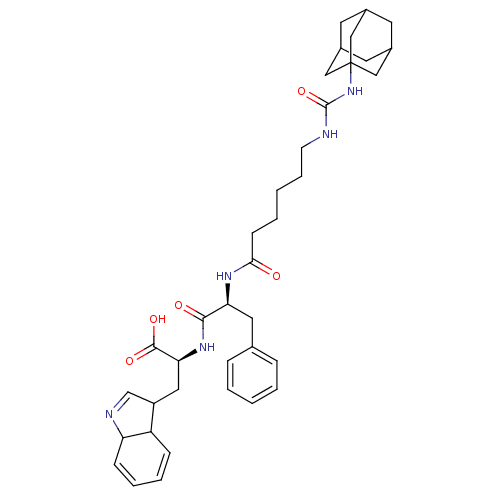 (CHEMBL385894 | N-[6-(3-adamantyl-ureido)-hexanoyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble Epoxide hydrolase | Bioorg Med Chem Lett 16: 5439-44 (2006) Article DOI: 10.1016/j.bmcl.2006.07.073 BindingDB Entry DOI: 10.7270/Q2183643 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50135653 (2,4,6,8,10,12-hexa(2-chloro phenylmethyl)-2,4,6,8,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against acetylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50135654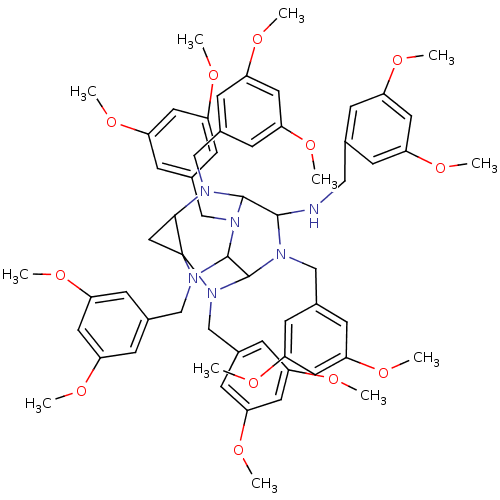 (2,4,6,8,10,12-hexa(3,5-dimethoxyphenylmethyl)-2,4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Butyrylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50135654 (2,4,6,8,10,12-hexa(3,5-dimethoxyphenylmethyl)-2,4,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against acetylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50135652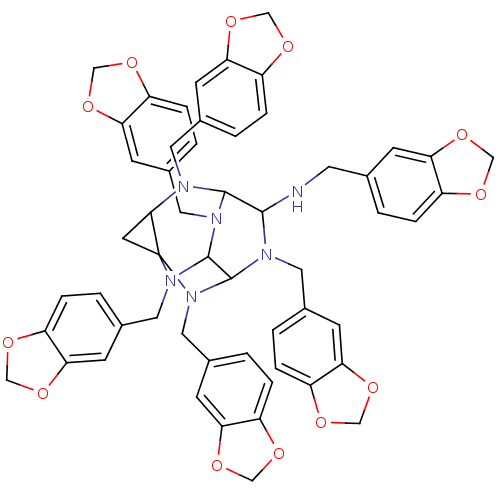 (2,6,8N,9,11,12-hexabenzo[d][1,3]dioxol-5-ylmethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Butyrylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50135652 (2,6,8N,9,11,12-hexabenzo[d][1,3]dioxol-5-ylmethyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against acetylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50135655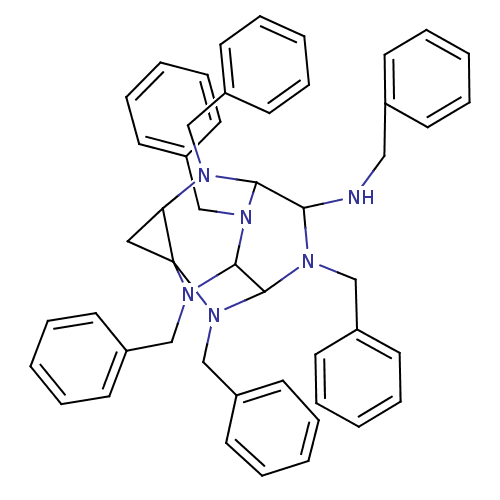 (2,4,6,8,10,12-Hexabenzyl-2,4,6,8,10,12-hexaazatetr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Butyrylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50135655 (2,4,6,8,10,12-Hexabenzyl-2,4,6,8,10,12-hexaazatetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against acetylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D820Y single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 phosphorylation at 0.1 to 1000 nM after 1 hr by... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 D835Y mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 D835Y phosphorylation at 0.1 to 10... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 ITD mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 ITD phosphorylation at 0.1 to 1000 n... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 Y823D single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 11/17 V560G/N822K double mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot k... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217458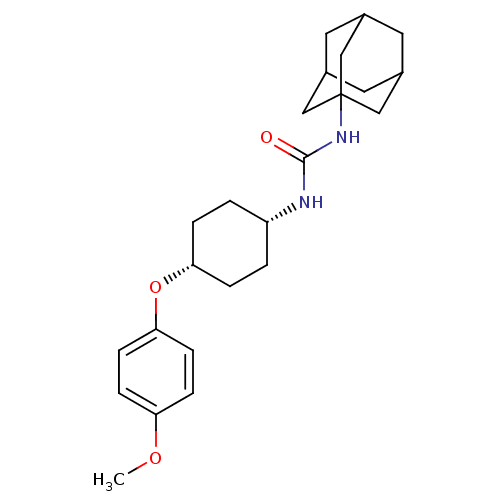 (CHEMBL244192 | cis-1-adamantan-1-yl-3-[4-(4-methox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217444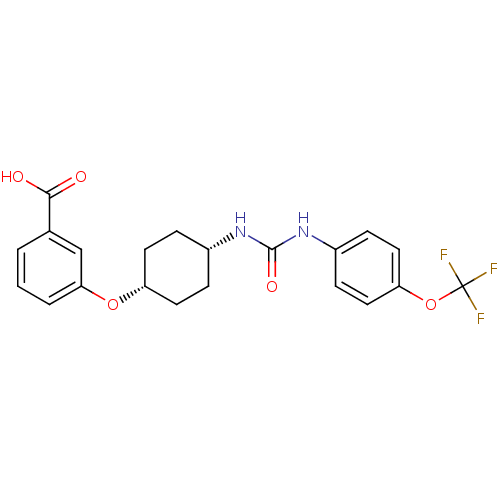 (CHEMBL244002 | cis-4-{4-[3-(4-trifluoromethoxyphen...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217457 (CHEMBL397694 | trans-1-adamantan-1-yl-3-[4-(4-nitr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D816H single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196664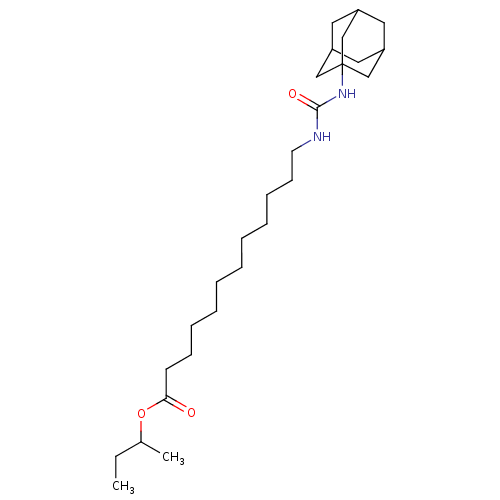 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid sec-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescent based assay | Bioorg Med Chem 15: 312-23 (2006) Article DOI: 10.1016/j.bmc.2006.09.057 BindingDB Entry DOI: 10.7270/Q26D5SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217469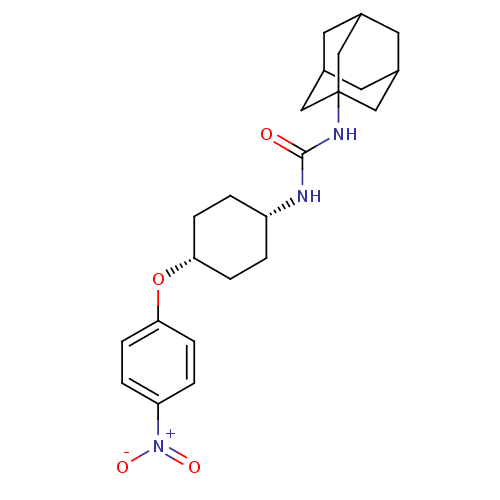 (CHEMBL244193 | cis-1-adamantan-1-yl-3-[4-(4-nitrop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217459 (CHEMBL243335 | trans-1-adamantan-1-yl-3-[4-(4-fluo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196661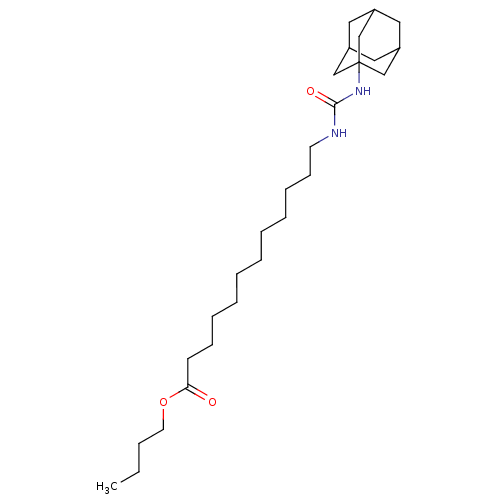 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescent based assay | Bioorg Med Chem 15: 312-23 (2006) Article DOI: 10.1016/j.bmc.2006.09.057 BindingDB Entry DOI: 10.7270/Q26D5SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D820E single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217446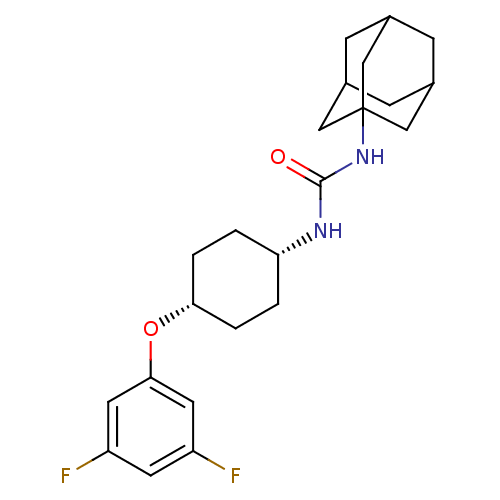 (CHEMBL396546 | cis-1-adamantan-1-yl-3-[4-(3,5-difl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217451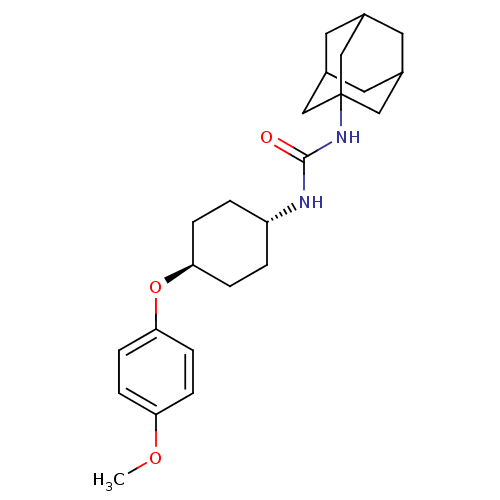 (CHEMBL427695 | trans-1-adamantan-1-yl-3-[4-(4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 18 A829P single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217439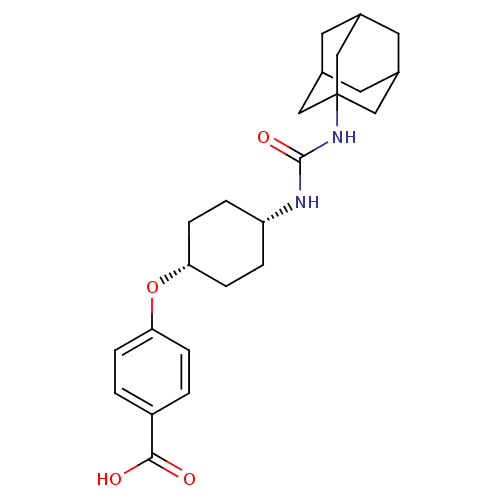 (CHEMBL244405 | cis-4-[4-(3-adamantan-1-ylureido)cy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50194498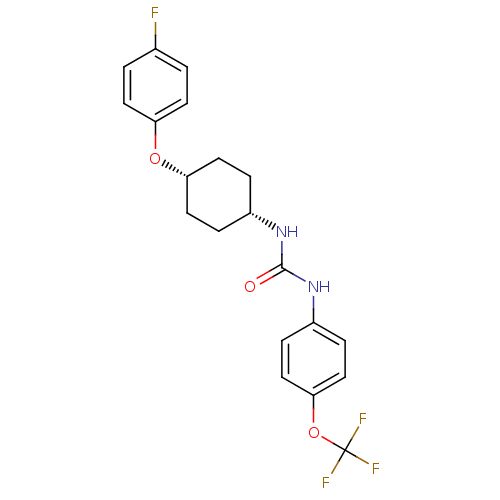 (1-[4-(4-fluoro-phenoxy)-cyclohexyl]-3-(4-trifluoro...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217477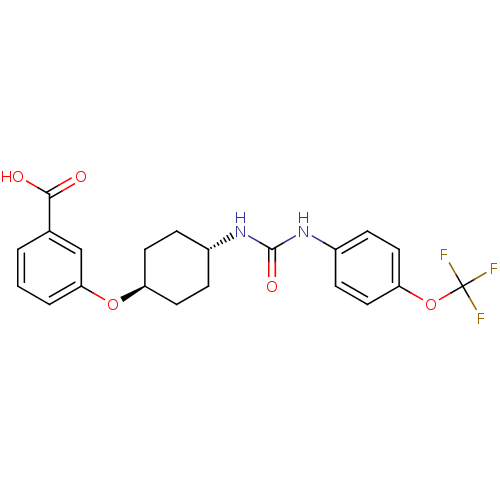 (CHEMBL243793 | trans-4-{4-[3-(4-trifluoromethoxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217461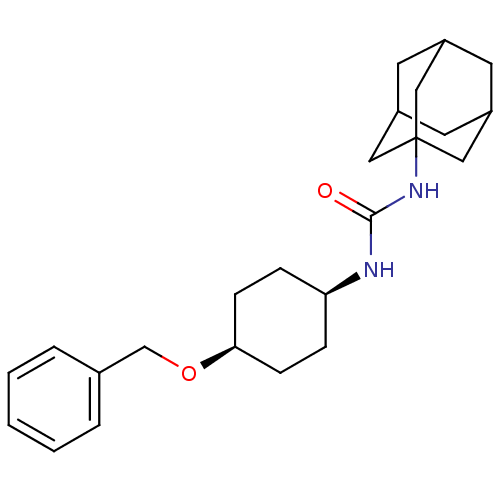 (CHEMBL242898 | cis-1-adamantan-1-yl-3-(4-benzyloxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT JM domain exon 11 V560G single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217473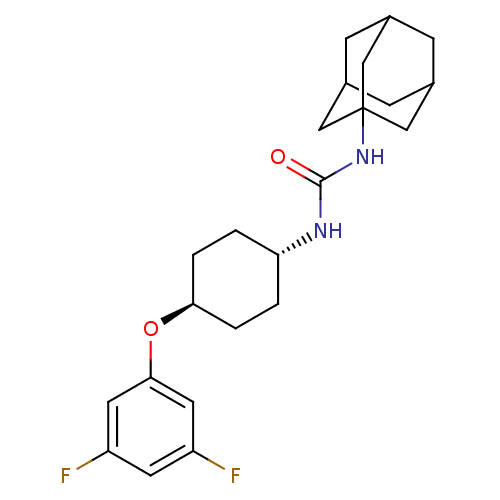 (CHEMBL243336 | trans-1-adamantan-1-yl-3-[4-(3,5-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196655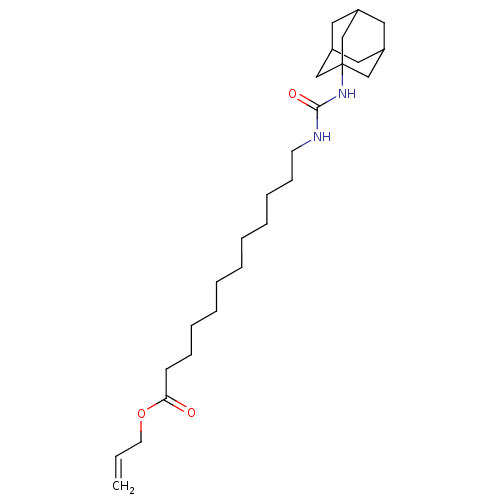 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid allyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescent based assay | Bioorg Med Chem 15: 312-23 (2006) Article DOI: 10.1016/j.bmc.2006.09.057 BindingDB Entry DOI: 10.7270/Q26D5SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50194500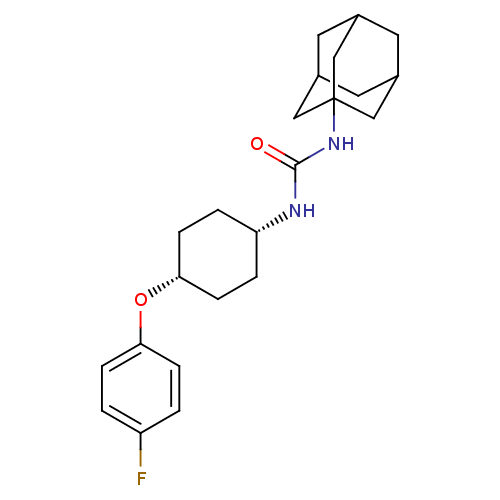 (1-adamantan-1-yl-3-[4-(4-fluoro-phenoxy)-cyclohexy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196654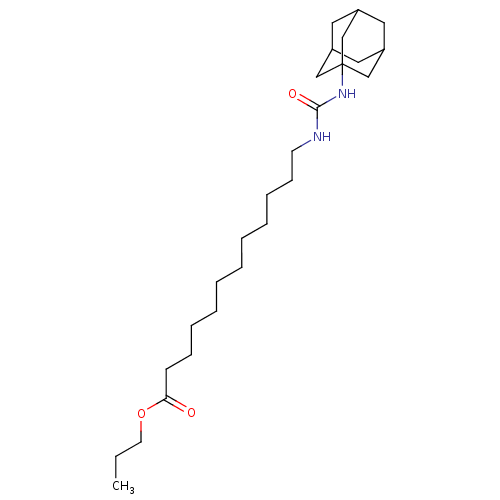 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescent based assay | Bioorg Med Chem 15: 312-23 (2006) Article DOI: 10.1016/j.bmc.2006.09.057 BindingDB Entry DOI: 10.7270/Q26D5SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217454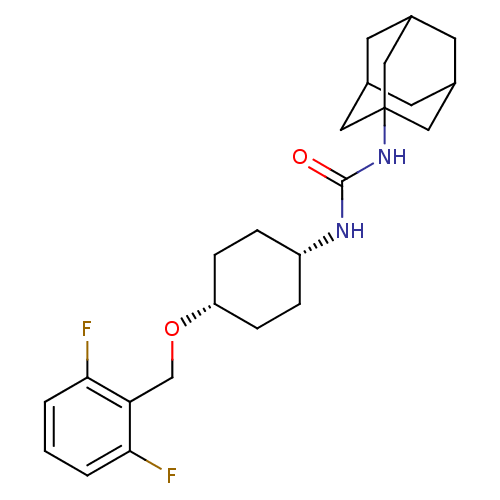 (CHEMBL395738 | cis-1-adamantan-1-yl-3-[4-(2,6-difl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50223391 (1-Adamantan-1-yl-3-{3-[2-(2-ethoxy-ethoxy)-ethoxy]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by fluorescent based assay | J Med Chem 50: 5217-26 (2007) Article DOI: 10.1021/jm070705c BindingDB Entry DOI: 10.7270/Q24T6J3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50191859 (1-adamantan-1-yl-3-[1-(2,2,2-trifluoro-acetyl)-pip...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay | Bioorg Med Chem Lett 16: 5212-6 (2006) Article DOI: 10.1016/j.bmcl.2006.07.009 BindingDB Entry DOI: 10.7270/Q29023DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196649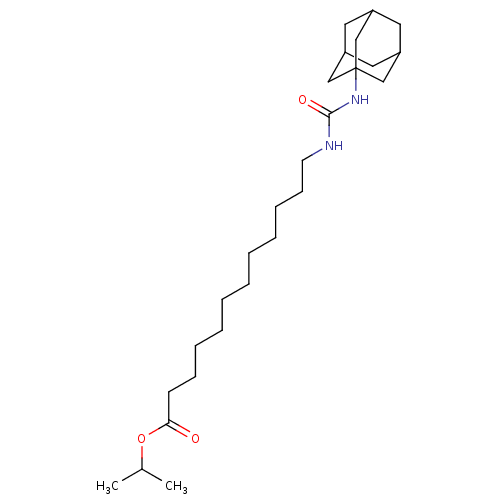 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid isopr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescent based assay | Bioorg Med Chem 15: 312-23 (2006) Article DOI: 10.1016/j.bmc.2006.09.057 BindingDB Entry DOI: 10.7270/Q26D5SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50223398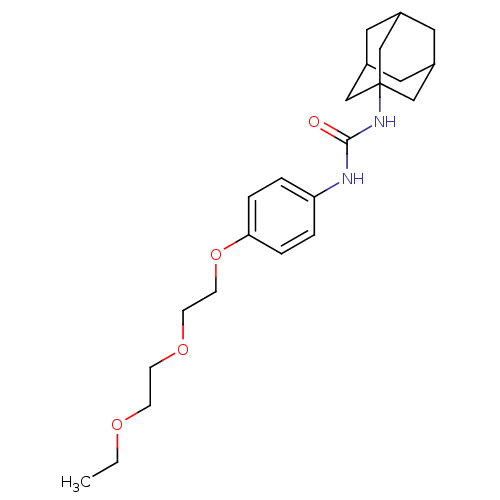 (1-Adamantan-1-yl-3-{4-[2-(2-ethoxy-ethoxy)-ethoxy]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescence assay | Bioorg Med Chem Lett 19: 1784-9 (2009) Article DOI: 10.1016/j.bmcl.2009.01.069 BindingDB Entry DOI: 10.7270/Q2ZP461N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50191898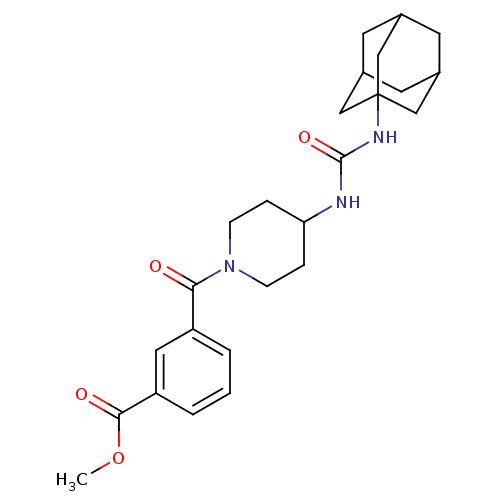 (3-[4-(3-adamantan-1-yl-ureido)-piperidine-1-carbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay | Bioorg Med Chem Lett 16: 5212-6 (2006) Article DOI: 10.1016/j.bmcl.2006.07.009 BindingDB Entry DOI: 10.7270/Q29023DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50191882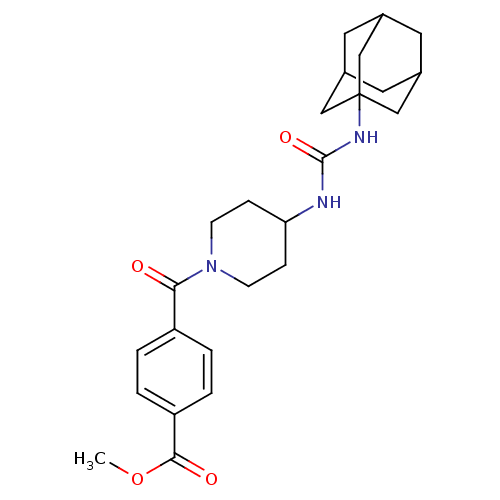 (4-[4-(3-adamantan-1-yl-ureido)-piperidine-1-carbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay | Bioorg Med Chem Lett 16: 5212-6 (2006) Article DOI: 10.1016/j.bmcl.2006.07.009 BindingDB Entry DOI: 10.7270/Q29023DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50223384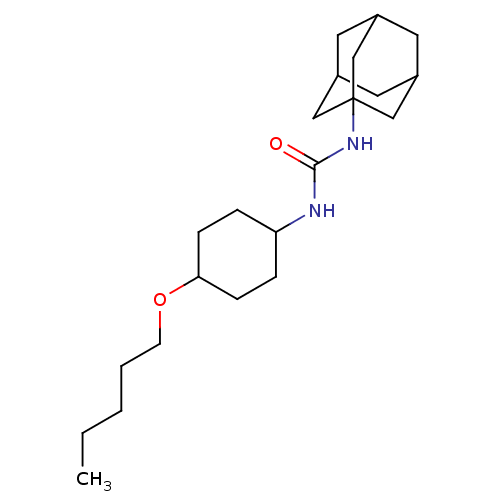 (1-adamantan-1-yl-3-(4-pentyloxycylclohexyl)urea | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by fluorescent based assay | J Med Chem 50: 5217-26 (2007) Article DOI: 10.1021/jm070705c BindingDB Entry DOI: 10.7270/Q24T6J3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50191867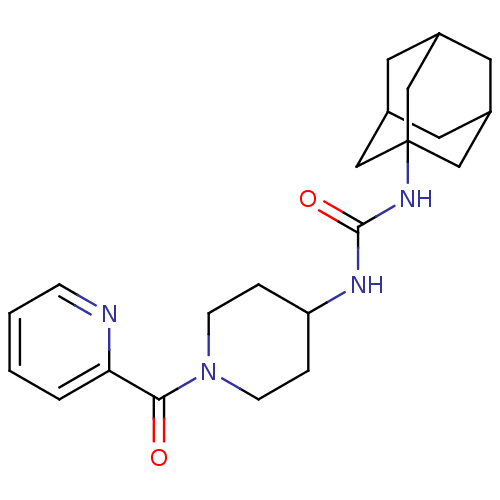 (1-adamantan-1-yl-3-[1-(pyridine-2-carbonyl)-piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay | Bioorg Med Chem Lett 16: 5212-6 (2006) Article DOI: 10.1016/j.bmcl.2006.07.009 BindingDB Entry DOI: 10.7270/Q29023DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196660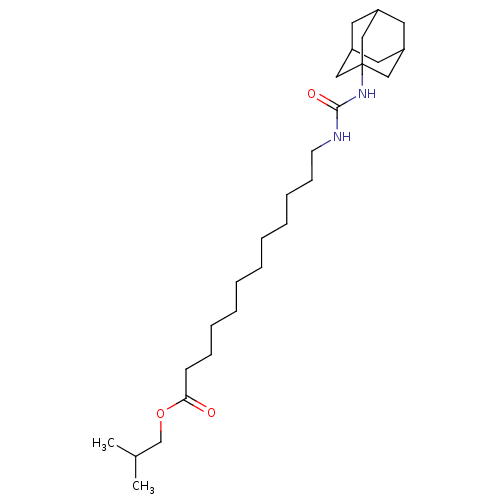 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid isobu...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescent based assay | Bioorg Med Chem 15: 312-23 (2006) Article DOI: 10.1016/j.bmc.2006.09.057 BindingDB Entry DOI: 10.7270/Q26D5SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50223389 (1-Adamantan-1-yl-3-[4-(3-morpholin-4-yl-propoxy)-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by fluorescent based assay | J Med Chem 50: 5217-26 (2007) Article DOI: 10.1021/jm070705c BindingDB Entry DOI: 10.7270/Q24T6J3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196658 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid tert-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by fluorescent based assay | Bioorg Med Chem 15: 312-23 (2006) Article DOI: 10.1016/j.bmc.2006.09.057 BindingDB Entry DOI: 10.7270/Q26D5SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217456 (CHEMBL243337 | cis-1-adamantan-1-yl-3-[4-(4-bromop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217448 (CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase | J Med Chem 50: 3825-40 (2007) Article DOI: 10.1021/jm070270t BindingDB Entry DOI: 10.7270/Q2TQ62BW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 493 total ) | Next | Last >> |