 Found 160 hits with Last Name = 'do' and Initial = 'zn'
Found 160 hits with Last Name = 'do' and Initial = 'zn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296224

(1-(4-(3-morpholinopropoxy)phenyl)-3-(4-(trifluorom...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)Nc2ccc(OCCCN3CCOCC3)cc2)cc1 Show InChI InChI=1S/C21H24F3N3O4/c22-21(23,24)31-19-8-4-17(5-9-19)26-20(28)25-16-2-6-18(7-3-16)30-13-1-10-27-11-14-29-15-12-27/h2-9H,1,10-15H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276625

(1-Adamantan-1-yl-3-[3-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(Nc1cccc(OCCCN2CCOCC2)c1)NC12CC3CC(CC(C3)C1)C2 |TLB:19:20:23:27.26.25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20| Show InChI InChI=1S/C24H35N3O3/c28-23(26-24-15-18-11-19(16-24)13-20(12-18)17-24)25-21-3-1-4-22(14-21)30-8-2-5-27-6-9-29-10-7-27/h1,3-4,14,18-20H,2,5-13,15-17H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296223
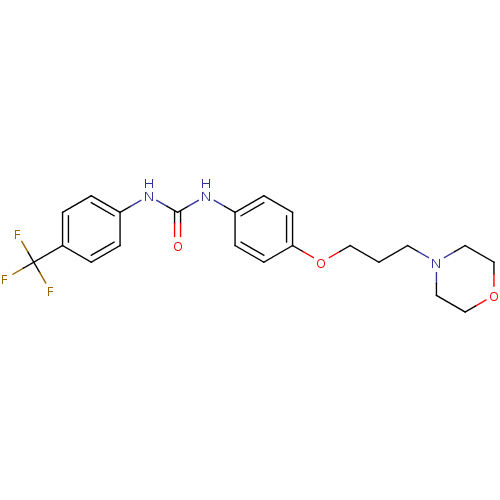
(1-(4-(3-morpholinopropoxy)phenyl)-3-(4-(trifluorom...)Show SMILES FC(F)(F)c1ccc(NC(=O)Nc2ccc(OCCCN3CCOCC3)cc2)cc1 Show InChI InChI=1S/C21H24F3N3O3/c22-21(23,24)16-2-4-17(5-3-16)25-20(28)26-18-6-8-19(9-7-18)30-13-1-10-27-11-14-29-15-12-27/h2-9H,1,10-15H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296214

(1-(4-fluoro-3-(3-morpholinopropoxy)phenyl)-3-(4-(t...)Show SMILES Fc1ccc(NC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1OCCCN1CCOCC1 Show InChI InChI=1S/C21H23F4N3O4/c22-18-7-4-16(14-19(18)31-11-1-8-28-9-12-30-13-10-28)27-20(29)26-15-2-5-17(6-3-15)32-21(23,24)25/h2-7,14H,1,8-13H2,(H2,26,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296210
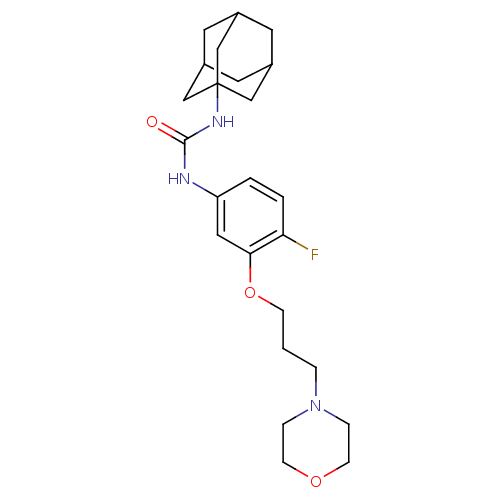
(1-Adamantan-1-yl-3-[4-fluoro-3-(3-morpholin-4-yl-p...)Show SMILES Fc1ccc(NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1OCCCN1CCOCC1 |TLB:8:9:12:16.15.14,THB:10:11:14:18.9.17,10:9:12.11.16:14,17:9:12:16.15.14,17:15:12:18.10.9| Show InChI InChI=1S/C24H34FN3O3/c25-21-3-2-20(13-22(21)31-7-1-4-28-5-8-30-9-6-28)26-23(29)27-24-14-17-10-18(15-24)12-19(11-17)16-24/h2-3,13,17-19H,1,4-12,14-16H2,(H2,26,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25743

(1-cycloheptyl-3-(1-acetylpiperidin-4-yl)urea | US8...)Show InChI InChI=1S/C15H27N3O2/c1-12(19)18-10-8-14(9-11-18)17-15(20)16-13-6-4-2-3-5-7-13/h13-14H,2-11H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276625

(1-Adamantan-1-yl-3-[3-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(Nc1cccc(OCCCN2CCOCC2)c1)NC12CC3CC(CC(C3)C1)C2 |TLB:19:20:23:27.26.25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20| Show InChI InChI=1S/C24H35N3O3/c28-23(26-24-15-18-11-19(16-24)13-20(12-18)17-24)25-21-3-1-4-22(14-21)30-8-2-5-27-6-9-29-10-7-27/h1,3-4,14,18-20H,2,5-13,15-17H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276673

(1-(3-(3-morpholinopropoxy)phenyl)-3-(4-(trifluorom...)Show SMILES FC(F)(F)c1ccc(NC(=O)Nc2cccc(OCCCN3CCOCC3)c2)cc1 Show InChI InChI=1S/C21H24F3N3O3/c22-21(23,24)16-5-7-17(8-6-16)25-20(28)26-18-3-1-4-19(15-18)30-12-2-9-27-10-13-29-14-11-27/h1,3-8,15H,2,9-14H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191898
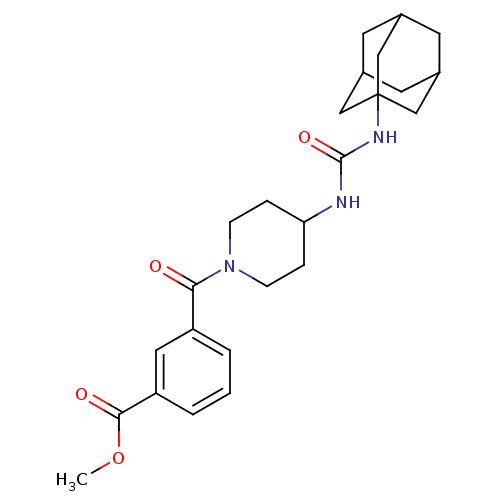
(3-[4-(3-adamantan-1-yl-ureido)-piperidine-1-carbon...)Show SMILES COC(=O)c1cccc(c1)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:21:22:25.24.29:27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22,21:22:25:29.28.27| Show InChI InChI=1S/C25H33N3O4/c1-32-23(30)20-4-2-3-19(12-20)22(29)28-7-5-21(6-8-28)26-24(31)27-25-13-16-9-17(14-25)11-18(10-16)15-25/h2-4,12,16-18,21H,5-11,13-15H2,1H3,(H2,26,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191882
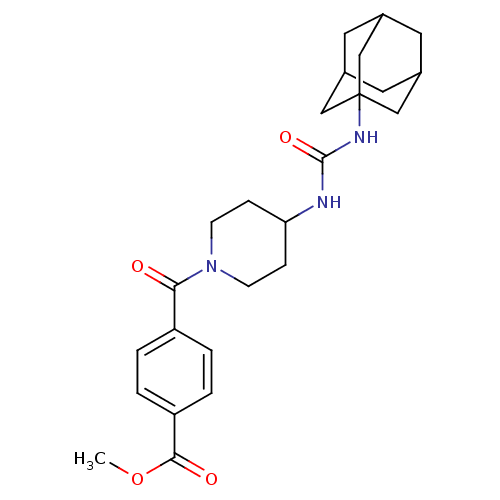
(4-[4-(3-adamantan-1-yl-ureido)-piperidine-1-carbon...)Show SMILES COC(=O)c1ccc(cc1)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:21:22:25.24.29:27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22,21:22:25:29.28.27| Show InChI InChI=1S/C25H33N3O4/c1-32-23(30)20-4-2-19(3-5-20)22(29)28-8-6-21(7-9-28)26-24(31)27-25-13-16-10-17(14-25)12-18(11-16)15-25/h2-5,16-18,21H,6-15H2,1H3,(H2,26,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191859

(1-adamantan-1-yl-3-[1-(2,2,2-trifluoro-acetyl)-pip...)Show SMILES FC(F)(F)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:15:16:19.18.23:21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,15:16:19:23.22.21| Show InChI InChI=1S/C18H26F3N3O2/c19-18(20,21)15(25)24-3-1-14(2-4-24)22-16(26)23-17-8-11-5-12(9-17)7-13(6-11)10-17/h11-14H,1-10H2,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276673

(1-(3-(3-morpholinopropoxy)phenyl)-3-(4-(trifluorom...)Show SMILES FC(F)(F)c1ccc(NC(=O)Nc2cccc(OCCCN3CCOCC3)c2)cc1 Show InChI InChI=1S/C21H24F3N3O3/c22-21(23,24)16-5-7-17(8-6-16)25-20(28)26-18-3-1-4-19(15-18)30-12-2-9-27-10-13-29-14-11-27/h1,3-8,15H,2,9-14H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191867
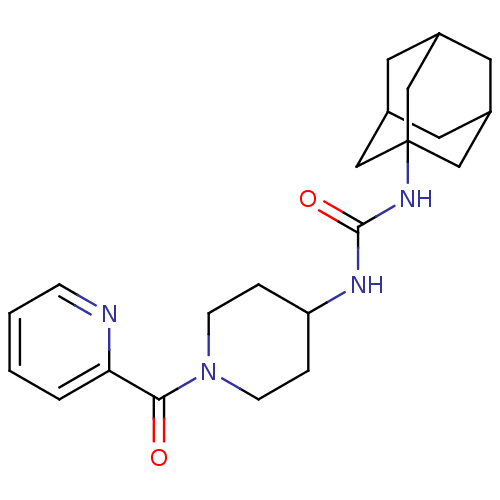
(1-adamantan-1-yl-3-[1-(pyridine-2-carbonyl)-piperi...)Show SMILES O=C(NC1CCN(CC1)C(=O)c1ccccn1)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23| Show InChI InChI=1S/C22H30N4O2/c27-20(19-3-1-2-6-23-19)26-7-4-18(5-8-26)24-21(28)25-22-12-15-9-16(13-22)11-17(10-15)14-22/h1-3,6,15-18H,4-5,7-14H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296226

(2-Adamantan-1-yl-N-[4-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(CC12CC3CC(CC(C3)C1)C2)Nc1ccc(OCCCN2CCOCC2)cc1 |TLB:2:3:6:10.9.8,THB:4:5:8:12.3.11,4:3:6.5.10:8,11:3:6:10.9.8,11:9:6:12.4.3| Show InChI InChI=1S/C25H36N2O3/c28-24(18-25-15-19-12-20(16-25)14-21(13-19)17-25)26-22-2-4-23(5-3-22)30-9-1-6-27-7-10-29-11-8-27/h2-5,19-21H,1,6-18H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191865

(1-adamantan-1-yl-3-(1-benzoyl-piperidin-4-yl)-urea...)Show SMILES O=C(NC1CCN(CC1)C(=O)c1ccccc1)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23| Show InChI InChI=1S/C23H31N3O2/c27-21(19-4-2-1-3-5-19)26-8-6-20(7-9-26)24-22(28)25-23-13-16-10-17(14-23)12-18(11-16)15-23/h1-5,16-18,20H,6-15H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296213

(1-(4-fluoro-3-(3-morpholinopropoxy)phenyl)-3-(4-(t...)Show SMILES Fc1ccc(NC(=O)Nc2ccc(cc2)C(F)(F)F)cc1OCCCN1CCOCC1 Show InChI InChI=1S/C21H23F4N3O3/c22-18-7-6-17(14-19(18)31-11-1-8-28-9-12-30-13-10-28)27-20(29)26-16-4-2-15(3-5-16)21(23,24)25/h2-7,14H,1,8-13H2,(H2,26,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50223389

(1-Adamantan-1-yl-3-[4-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(Nc1ccc(OCCCN2CCOCC2)cc1)NC12CC3CC(CC(C3)C1)C2 |TLB:19:20:23.22.27:25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20,19:20:23:27.26.25| Show InChI InChI=1S/C24H35N3O3/c28-23(26-24-15-18-12-19(16-24)14-20(13-18)17-24)25-21-2-4-22(5-3-21)30-9-1-6-27-7-10-29-11-8-27/h2-5,18-20H,1,6-17H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191857

(4-{4-[(3-adamantan-1-yl-ureido)-methyl]-piperidine...)Show SMILES COC(=O)c1ccc(cc1)C(=O)N1CCC(CNC(=O)NC23CC4CC(CC(C4)C2)C3)CC1 |TLB:20:21:24.23.28:26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21,20:21:24:28.27.26| Show InChI InChI=1S/C26H35N3O4/c1-33-24(31)22-4-2-21(3-5-22)23(30)29-8-6-17(7-9-29)16-27-25(32)28-26-13-18-10-19(14-26)12-20(11-18)15-26/h2-5,17-20H,6-16H2,1H3,(H2,27,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296225

(1-(4-chlorophenyl)-3-(4-(3-morpholinopropoxy)pheny...)Show InChI InChI=1S/C20H24ClN3O3/c21-16-2-4-17(5-3-16)22-20(25)23-18-6-8-19(9-7-18)27-13-1-10-24-11-14-26-15-12-24/h2-9H,1,10-15H2,(H2,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335965
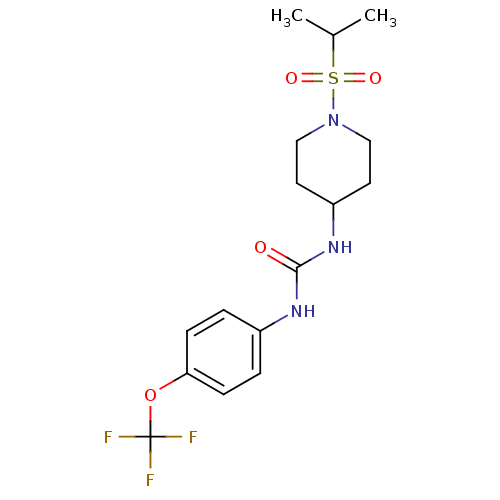
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H22F3N3O4S/c1-11(2)27(24,25)22-9-7-13(8-10-22)21-15(23)20-12-3-5-14(6-4-12)26-16(17,18)19/h3-6,11,13H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335964

(1-(1-nicotinoylpiperidin-4-yl)-3-(4-(trifluorometh...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H19F3N4O3/c20-19(21,22)29-16-5-3-14(4-6-16)24-18(28)25-15-7-10-26(11-8-15)17(27)13-2-1-9-23-12-13/h1-6,9,12,15H,7-8,10-11H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191880
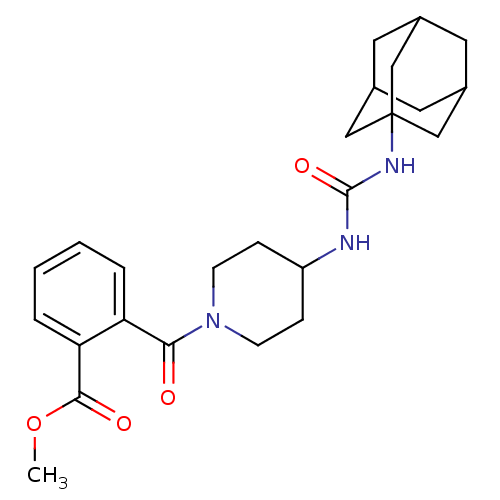
(2-[4-(3-adamantan-1-yl-ureido)-piperidine-1-carbon...)Show SMILES COC(=O)c1ccccc1C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:21:22:25.24.29:27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22,21:22:25:29.28.27| Show InChI InChI=1S/C25H33N3O4/c1-32-23(30)21-5-3-2-4-20(21)22(29)28-8-6-19(7-9-28)26-24(31)27-25-13-16-10-17(14-25)12-18(11-16)15-25/h2-5,16-19H,6-15H2,1H3,(H2,26,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191897
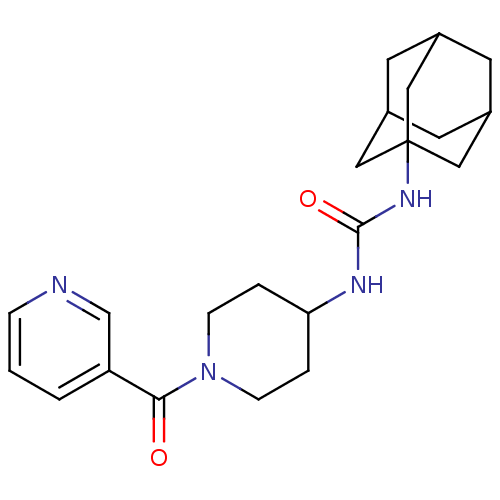
(1-adamantan-1-yl-3-[1-(pyridine-3-carbonyl)-piperi...)Show SMILES O=C(NC1CCN(CC1)C(=O)c1cccnc1)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23| Show InChI InChI=1S/C22H30N4O2/c27-20(18-2-1-5-23-14-18)26-6-3-19(4-7-26)24-21(28)25-22-11-15-8-16(12-22)10-17(9-15)13-22/h1-2,5,14-17,19H,3-4,6-13H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191893
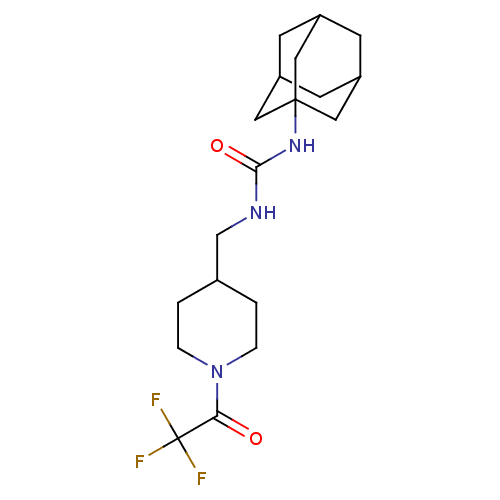
(1-adamantan-1-yl-3-[1-(2,2,2-trifluoro-acetyl)-pip...)Show SMILES FC(F)(F)C(=O)N1CCC(CNC(=O)NC23CC4CC(CC(C4)C2)C3)CC1 |TLB:14:15:18.17.22:20,THB:16:17:20:24.15.23,16:15:18.17.22:20,23:15:18:22.21.20,23:21:18:24.16.15,14:15:18:22.21.20| Show InChI InChI=1S/C19H28F3N3O2/c20-19(21,22)16(26)25-3-1-12(2-4-25)11-23-17(27)24-18-8-13-5-14(9-18)7-15(6-13)10-18/h12-15H,1-11H2,(H2,23,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191870
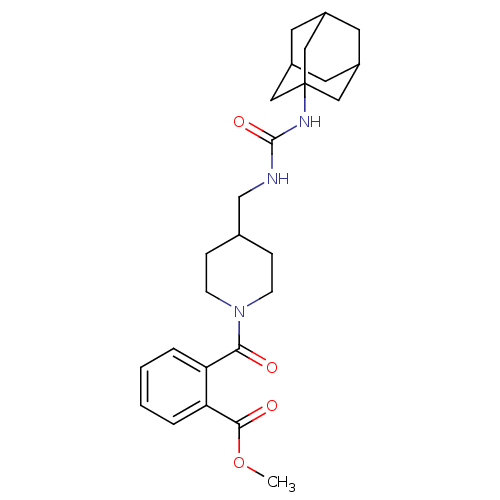
(2-{4-[(3-adamantan-1-yl-ureido)-methyl]-piperidine...)Show SMILES COC(=O)c1ccccc1C(=O)N1CCC(CNC(=O)NC23CC4CC(CC(C4)C2)C3)CC1 |TLB:20:21:24.23.28:26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21,20:21:24:28.27.26| Show InChI InChI=1S/C26H35N3O4/c1-33-24(31)22-5-3-2-4-21(22)23(30)29-8-6-17(7-9-29)16-27-25(32)28-26-13-18-10-19(14-26)12-20(11-18)15-26/h2-5,17-20H,6-16H2,1H3,(H2,27,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966
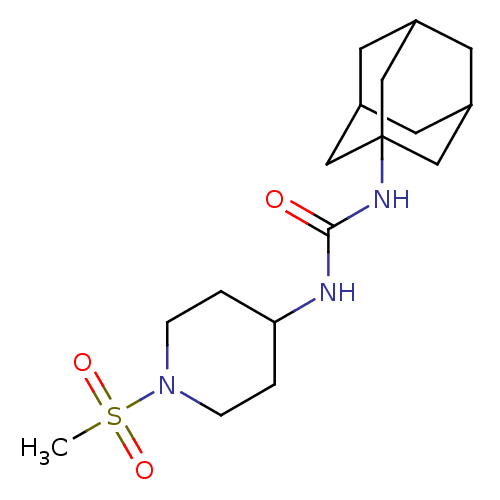
(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296197
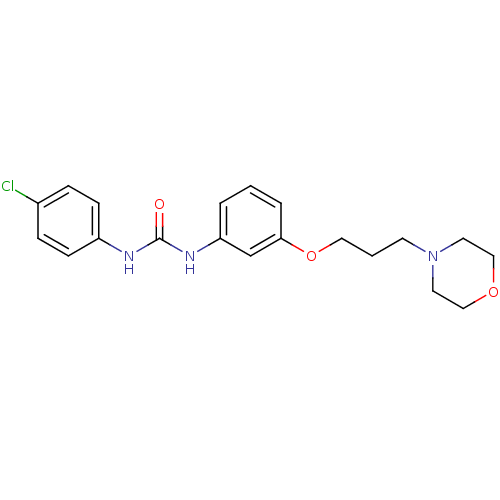
(1-(4-chlorophenyl)-3-(3-(3-morpholinopropoxy)pheny...)Show InChI InChI=1S/C20H24ClN3O3/c21-16-5-7-17(8-6-16)22-20(25)23-18-3-1-4-19(15-18)27-12-2-9-24-10-13-26-14-11-24/h1,3-8,15H,2,9-14H2,(H2,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191853
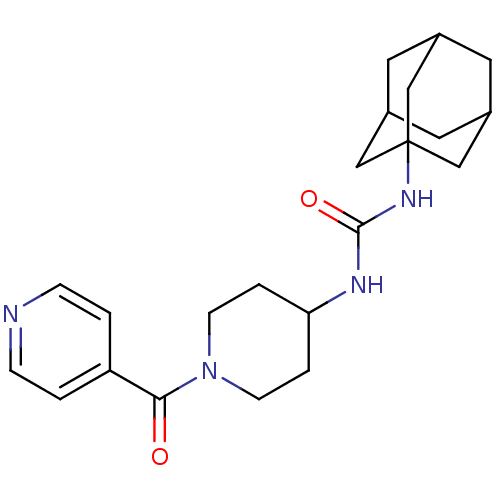
(1-adamantan-1-yl-3-[1-(pyridine-4-carbonyl)-piperi...)Show SMILES O=C(NC1CCN(CC1)C(=O)c1ccncc1)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23| Show InChI InChI=1S/C22H30N4O2/c27-20(18-1-5-23-6-2-18)26-7-3-19(4-8-26)24-21(28)25-22-12-15-9-16(13-22)11-17(10-15)14-22/h1-2,5-6,15-17,19H,3-4,7-14H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276676

(2-Adamantan-1-yl-N-[3-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(CC12CC3CC(CC(C3)C1)C2)Nc1cccc(OCCCN2CCOCC2)c1 |TLB:2:3:6:10.9.8,THB:4:5:8:12.3.11,4:3:6.5.10:8,11:3:6:10.9.8,11:9:6:12.4.3| Show InChI InChI=1S/C25H36N2O3/c28-24(18-25-15-19-11-20(16-25)13-21(12-19)17-25)26-22-3-1-4-23(14-22)30-8-2-5-27-6-9-29-10-7-27/h1,3-4,14,19-21H,2,5-13,15-18H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276676

(2-Adamantan-1-yl-N-[3-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(CC12CC3CC(CC(C3)C1)C2)Nc1cccc(OCCCN2CCOCC2)c1 |TLB:2:3:6:10.9.8,THB:4:5:8:12.3.11,4:3:6.5.10:8,11:3:6:10.9.8,11:9:6:12.4.3| Show InChI InChI=1S/C25H36N2O3/c28-24(18-25-15-19-11-20(16-25)13-21(12-19)17-25)26-22-3-1-4-23(14-22)30-8-2-5-27-6-9-29-10-7-27/h1,3-4,14,19-21H,2,5-13,15-18H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296219

(1-Adamantan-1-yl-3-[3-(3-morpholin-4-yl-propoxy)-c...)Show SMILES O=C(NC1CCCC(C1)OCCCN1CCOCC1)NC12CC3CC(CC(C3)C1)C2 |TLB:19:20:23:27.26.25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20| Show InChI InChI=1S/C24H41N3O3/c28-23(26-24-15-18-11-19(16-24)13-20(12-18)17-24)25-21-3-1-4-22(14-21)30-8-2-5-27-6-9-29-10-7-27/h18-22H,1-17H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191862
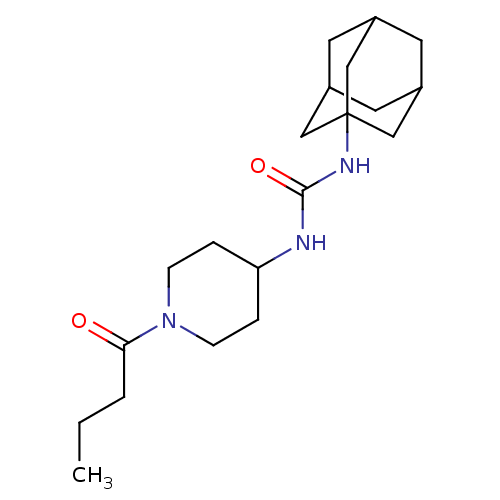
(1-adamantan-1-yl-3-(1-butyryl-piperidin-4-yl)-urea...)Show SMILES CCCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:14:15:18.17.22:20,THB:16:17:20:24.15.23,16:15:18.17.22:20,23:15:18:22.21.20,23:21:18:24.16.15,14:15:18:22.21.20| Show InChI InChI=1S/C20H33N3O2/c1-2-3-18(24)23-6-4-17(5-7-23)21-19(25)22-20-11-14-8-15(12-20)10-16(9-14)13-20/h14-17H,2-13H2,1H3,(H2,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191858
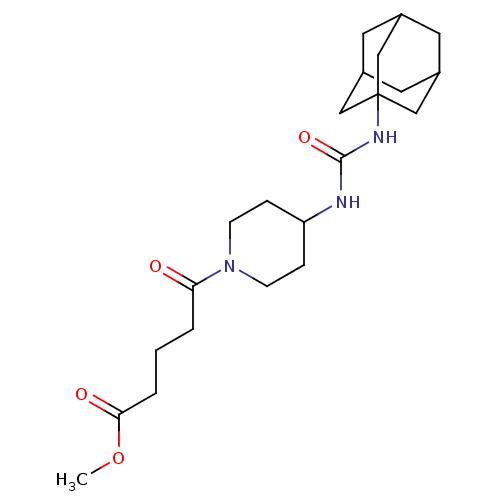
(5-[4-(3-adamantan-1-yl-ureido)-piperidin-1-yl]-5-o...)Show SMILES COC(=O)CCCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24| Show InChI InChI=1S/C22H35N3O4/c1-29-20(27)4-2-3-19(26)25-7-5-18(6-8-25)23-21(28)24-22-12-15-9-16(13-22)11-17(10-15)14-22/h15-18H,2-14H2,1H3,(H2,23,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335967
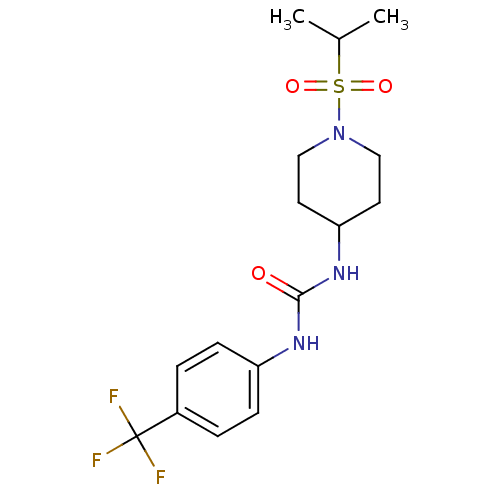
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H22F3N3O3S/c1-11(2)26(24,25)22-9-7-14(8-10-22)21-15(23)20-13-5-3-12(4-6-13)16(17,18)19/h3-6,11,14H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50191854
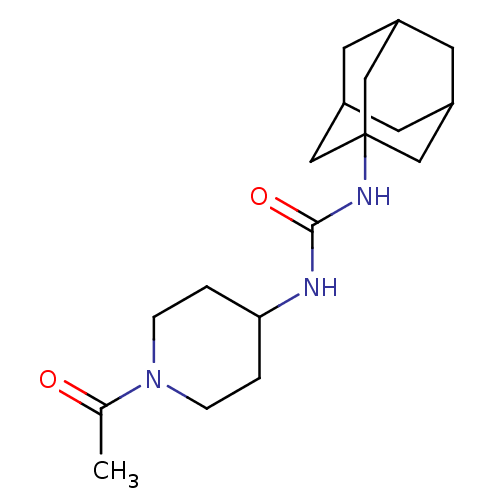
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191881
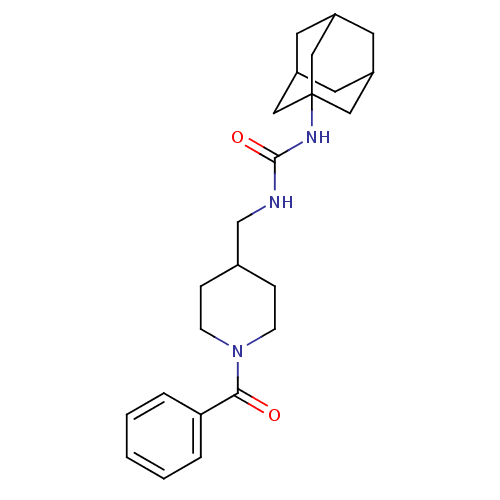
(1-adamantan-1-yl-3-(1-benzoyl-piperidin-4-ylmethyl...)Show SMILES O=C(NCC1CCN(CC1)C(=O)c1ccccc1)NC12CC3CC(CC(C3)C1)C2 |TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24| Show InChI InChI=1S/C24H33N3O2/c28-22(21-4-2-1-3-5-21)27-8-6-17(7-9-27)16-25-23(29)26-24-13-18-10-19(14-24)12-20(11-18)15-24/h1-5,17-20H,6-16H2,(H2,25,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191887

(1-adamantan-1-yl-3-(1-propionyl-piperidin-4-yl)-ur...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:13:14:17.16.21:19,THB:15:16:19:23.14.22,15:14:17.16.21:19,22:14:17:21.20.19,22:20:17:23.15.14,13:14:17:21.20.19| Show InChI InChI=1S/C19H31N3O2/c1-2-17(23)22-5-3-16(4-6-22)20-18(24)21-19-10-13-7-14(11-19)9-15(8-13)12-19/h13-16H,2-12H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191895
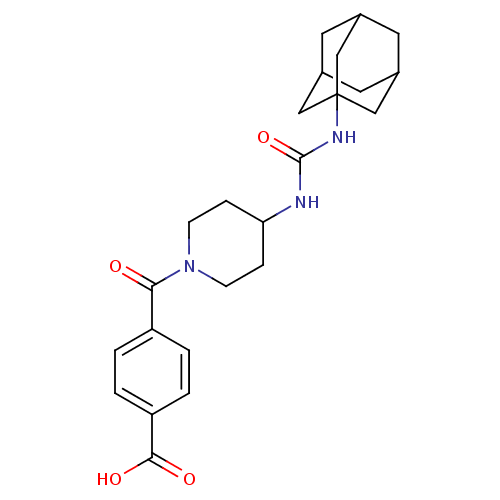
(4-[4-(3-adamantan-1-yl-ureido)-piperidine-1-carbon...)Show SMILES OC(=O)c1ccc(cc1)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:20:21:24.23.28:26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21,20:21:24:28.27.26| Show InChI InChI=1S/C24H31N3O4/c28-21(18-1-3-19(4-2-18)22(29)30)27-7-5-20(6-8-27)25-23(31)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-4,15-17,20H,5-14H2,(H,29,30)(H2,25,26,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191892
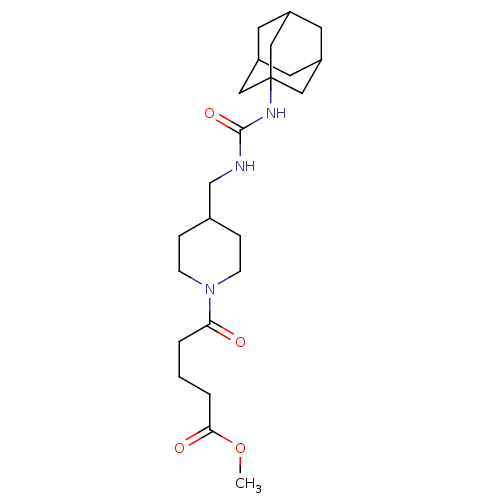
(5-{4-[(3-adamantan-1-yl-ureido)-methyl]-piperidin-...)Show SMILES COC(=O)CCCC(=O)N1CCC(CNC(=O)NC23CC4CC(CC(C4)C2)C3)CC1 |TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23| Show InChI InChI=1S/C23H37N3O4/c1-30-21(28)4-2-3-20(27)26-7-5-16(6-8-26)15-24-22(29)25-23-12-17-9-18(13-23)11-19(10-17)14-23/h16-19H,2-15H2,1H3,(H2,24,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335968
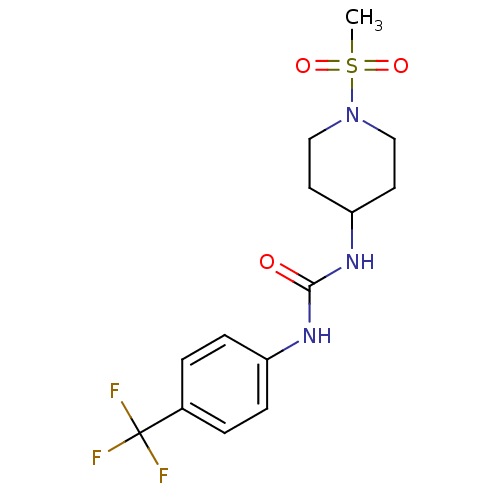
(1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluo...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H18F3N3O3S/c1-24(22,23)20-8-6-12(7-9-20)19-13(21)18-11-4-2-10(3-5-11)14(15,16)17/h2-5,12H,6-9H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276626
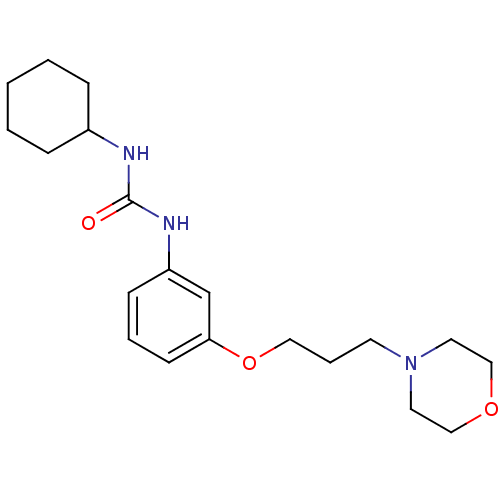
(1-cyclohexyl-3-(3-(3-morpholinopropoxy)phenyl)urea...)Show InChI InChI=1S/C20H31N3O3/c24-20(21-17-6-2-1-3-7-17)22-18-8-4-9-19(16-18)26-13-5-10-23-11-14-25-15-12-23/h4,8-9,16-17H,1-3,5-7,10-15H2,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276626

(1-cyclohexyl-3-(3-(3-morpholinopropoxy)phenyl)urea...)Show InChI InChI=1S/C20H31N3O3/c24-20(21-17-6-2-1-3-7-17)22-18-8-4-9-19(16-18)26-13-5-10-23-11-14-25-15-12-23/h4,8-9,16-17H,1-3,5-7,10-15H2,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966

(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191861
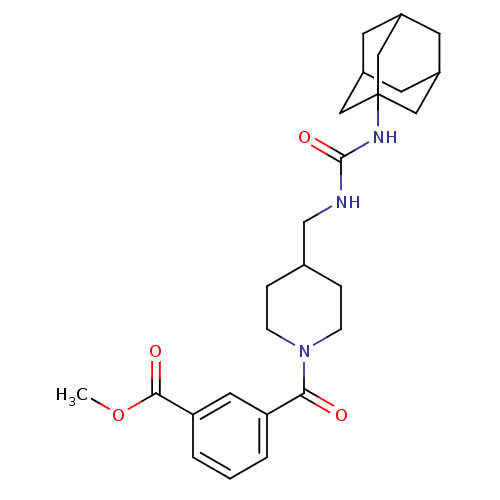
(3-{4-[(3-adamantan-1-yl-ureido)-methyl]-piperidine...)Show SMILES COC(=O)c1cccc(c1)C(=O)N1CCC(CNC(=O)NC23CC4CC(CC(C4)C2)C3)CC1 |TLB:20:21:24.23.28:26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21,20:21:24:28.27.26| Show InChI InChI=1S/C26H35N3O4/c1-33-24(31)22-4-2-3-21(12-22)23(30)29-7-5-17(6-8-29)16-27-25(32)28-26-13-18-9-19(14-26)11-20(10-18)15-26/h2-4,12,17-20H,5-11,13-16H2,1H3,(H2,27,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296215

(1-(4-chlorophenyl)-3-(4-fluoro-3-(3-morpholinoprop...)Show InChI InChI=1S/C20H23ClFN3O3/c21-15-2-4-16(5-3-15)23-20(26)24-17-6-7-18(22)19(14-17)28-11-1-8-25-9-12-27-13-10-25/h2-7,14H,1,8-13H2,(H2,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191878

(1-(1-Acetyl-piperidin-4-ylmethyl)-3-adamantan-1-yl...)Show SMILES CC(=O)N1CCC(CNC(=O)NC23CC4CC(CC(C4)C2)C3)CC1 |TLB:11:12:15.14.19:17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C19H31N3O2/c1-13(23)22-4-2-14(3-5-22)12-20-18(24)21-19-9-15-6-16(10-19)8-17(7-15)11-19/h14-17H,2-12H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296220
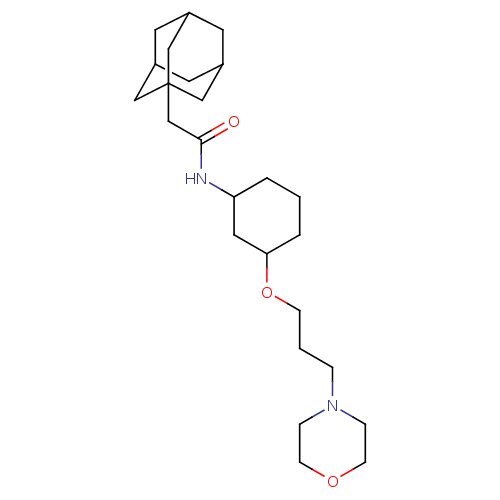
(2-Adamantan-1-yl-N-[3-(3-morpholin-4-yl-propoxy)-c...)Show SMILES O=C(CC12CC3CC(CC(C3)C1)C2)NC1CCCC(C1)OCCCN1CCOCC1 |TLB:2:3:6:10.9.8,THB:4:5:8:12.3.11,4:3:6.5.10:8,11:3:6:10.9.8,11:9:6:12.4.3| Show InChI InChI=1S/C25H42N2O3/c28-24(18-25-15-19-11-20(16-25)13-21(12-19)17-25)26-22-3-1-4-23(14-22)30-8-2-5-27-6-9-29-10-7-27/h19-23H,1-18H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191864

(1-adamantan-1-yl-3-[1-(pyridine-3-carbonyl)-piperi...)Show SMILES O=C(NCC1CCN(CC1)C(=O)c1cccnc1)NC12CC3CC(CC(C3)C1)C2 |TLB:18:19:22.21.26:24,THB:20:21:24:28.19.27,20:19:22.21.26:24,27:19:22:26.25.24,27:25:22:28.20.19,18:19:22:26.25.24| Show InChI InChI=1S/C23H32N4O2/c28-21(20-2-1-5-24-15-20)27-6-3-16(4-7-27)14-25-22(29)26-23-11-17-8-18(12-23)10-19(9-17)13-23/h1-2,5,15-19H,3-4,6-14H2,(H2,25,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydroxylase by kinetic fluorescent assay |
Bioorg Med Chem Lett 16: 5212-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.009
BindingDB Entry DOI: 10.7270/Q29023DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50296221
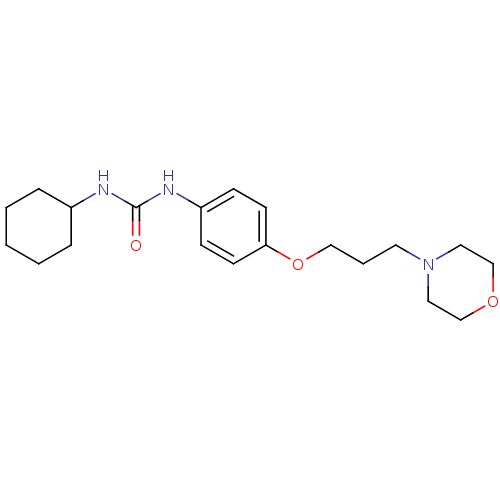
(1-cyclohexyl-3-(4-(3-morpholinopropoxy)phenyl)urea...)Show InChI InChI=1S/C20H31N3O3/c24-20(21-17-5-2-1-3-6-17)22-18-7-9-19(10-8-18)26-14-4-11-23-12-15-25-16-13-23/h7-10,17H,1-6,11-16H2,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Arete Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by fluorescent endpoint assay |
Bioorg Med Chem Lett 19: 4259-63 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.102
BindingDB Entry DOI: 10.7270/Q2VQ32Q0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data