| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Angiotensin-converting enzyme 2 |
|---|
| Ligand | BDBM50422743 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2270482 |
|---|
| Ki | 2.8±n/a nM |
|---|
| Citation |  Xiu, S; Dick, A; Ju, H; Mirzaie, S; Abdi, F; Cocklin, S; Zhan, P; Liu, X Inhibitors of SARS-CoV-2 Entry: Current and Future Opportunities. J Med Chem63:12256-12274 (2020) [PubMed] Xiu, S; Dick, A; Ju, H; Mirzaie, S; Abdi, F; Cocklin, S; Zhan, P; Liu, X Inhibitors of SARS-CoV-2 Entry: Current and Future Opportunities. J Med Chem63:12256-12274 (2020) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Angiotensin-converting enzyme 2 |
|---|
| Name: | Angiotensin-converting enzyme 2 |
|---|
| Synonyms: | ACE-related carboxypeptidase | ACE2 | ACE2_HUMAN | ACEH | Angiotensin-converting enzyme homolog | Angiotensin-converting enzyme-related carboxypeptidase | Metalloprotease MPROT15 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 92448.86 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 805 |
|---|
| Sequence: | MSSSSWLLLSLVAVTAAQSTIEEQAKTFLDKFNHEAEDLFYQSSLASWNYNTNITEENVQ
NMNNAGDKWSAFLKEQSTLAQMYPLQEIQNLTVKLQLQALQQNGSSVLSEDKSKRLNTIL
NTMSTIYSTGKVCNPDNPQECLLLEPGLNEIMANSLDYNERLWAWESWRSEVGKQLRPLY
EEYVVLKNEMARANHYEDYGDYWRGDYEVNGVDGYDYSRGQLIEDVEHTFEEIKPLYEHL
HAYVRAKLMNAYPSYISPIGCLPAHLLGDMWGRFWTNLYSLTVPFGQKPNIDVTDAMVDQ
AWDAQRIFKEAEKFFVSVGLPNMTQGFWENSMLTDPGNVQKAVCHPTAWDLGKGDFRILM
CTKVTMDDFLTAHHEMGHIQYDMAYAAQPFLLRNGANEGFHEAVGEIMSLSAATPKHLKS
IGLLSPDFQEDNETEINFLLKQALTIVGTLPFTYMLEKWRWMVFKGEIPKDQWMKKWWEM
KREIVGVVEPVPHDETYCDPASLFHVSNDYSFIRYYTRTLYQFQFQEALCQAAKHEGPLH
KCDISNSTEAGQKLFNMLRLGKSEPWTLALENVVGAKNMNVRPLLNYFEPLFTWLKDQNK
NSFVGWSTDWSPYADQSIKVRISLKSALGDKAYEWNDNEMYLFRSSVAYAMRQYFLKVKN
QMILFGEEDVRVANLKPRISFNFFVTAPKNVSDIIPRTEVEKAIRMSRSRINDAFRLNDN
SLEFLGIQPTLGPPNQPPVSIWLIVFGVVMGVIVVGIVILIFTGIRDRKKKNKARSGENP
YASIDISKGENNPGFQNTDDVQTSF
|
|
|
|---|
| BDBM50422743 |
|---|
| n/a |
|---|
| Name | BDBM50422743 |
|---|
| Synonyms: | CHEMBL5275219 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C139H184N34O40S2 |
|---|
| Mol. Mass. | 3035.282 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CO)NC(=O)[C@@H](CS)NC(=O)[C@@H](Cc1cnc[nH]1)NC(=O)[C@@H](CO)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CC(O)=O)NC(=O)CN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@@H]1C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CCCCN)C(=O)N[C@H](CS)C(=O)N[C@H]([C@H](C)O)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@@H]1C(=O)N[C@H](CC(O)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](CCC(O)=O)C(=O)NCC(=O)NCC(=O)NCC(O)=O |r| |
|---|
| Structure | 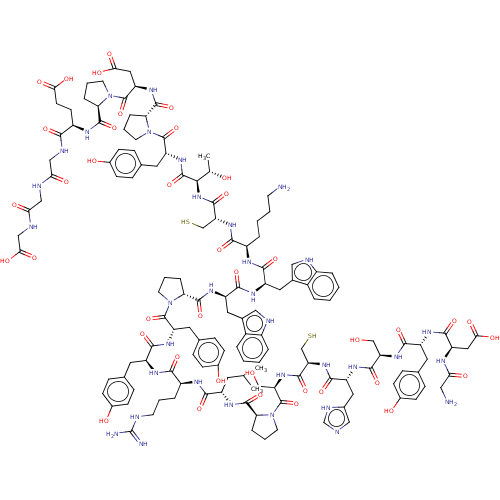
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xiu, S; Dick, A; Ju, H; Mirzaie, S; Abdi, F; Cocklin, S; Zhan, P; Liu, X Inhibitors of SARS-CoV-2 Entry: Current and Future Opportunities. J Med Chem63:12256-12274 (2020) [PubMed]
Xiu, S; Dick, A; Ju, H; Mirzaie, S; Abdi, F; Cocklin, S; Zhan, P; Liu, X Inhibitors of SARS-CoV-2 Entry: Current and Future Opportunities. J Med Chem63:12256-12274 (2020) [PubMed]