

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM14361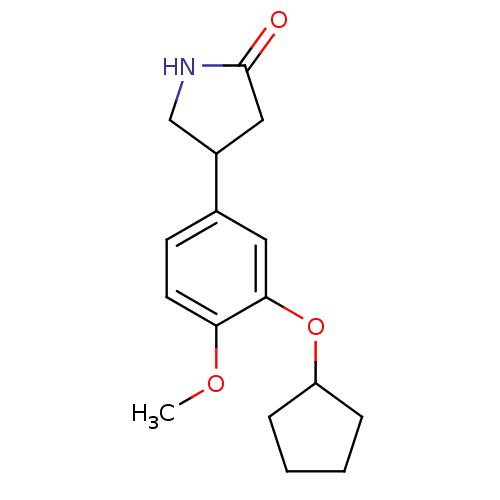 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111110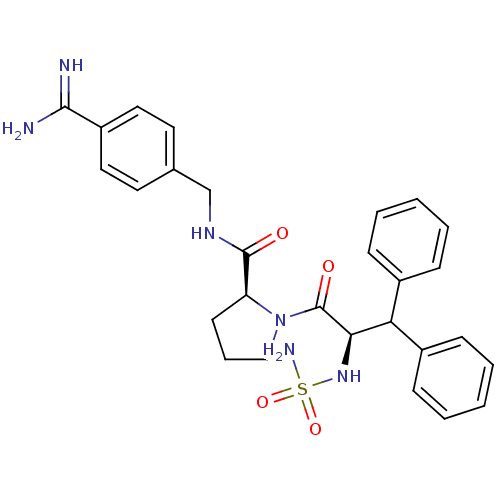 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111101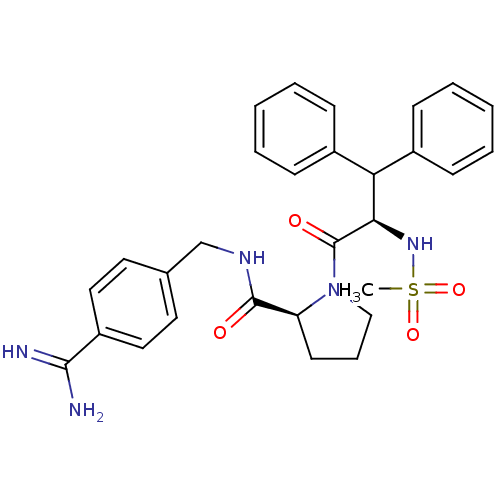 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111110 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111101 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111121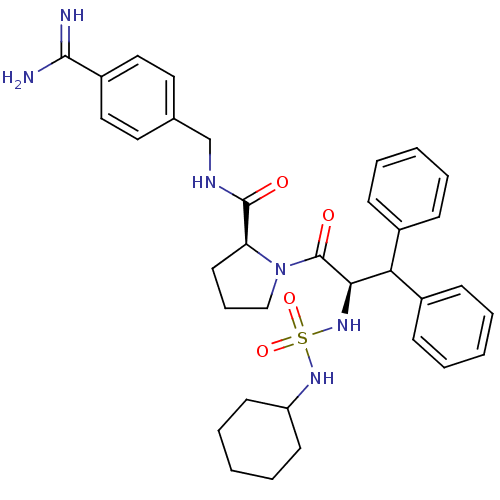 (2N-(4-Benzamidinemethyl)-1-[2-Cyclohexylaminosulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131789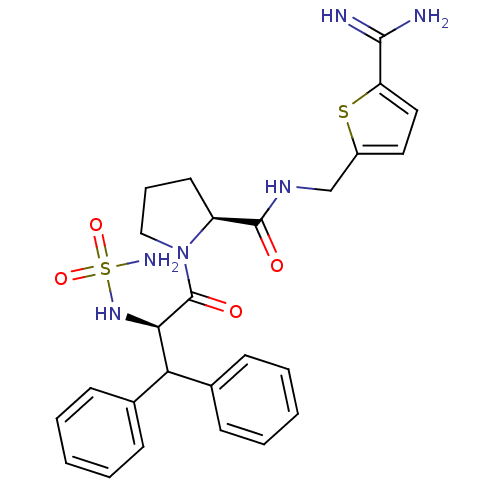 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111108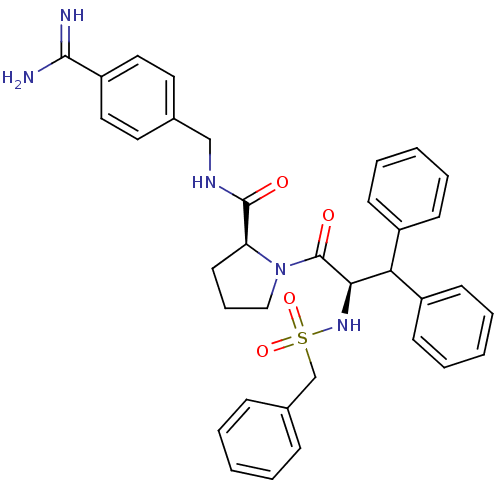 (2N-(4-Benzamidinemethyl)-1-[2-Benzylsulfonamido-3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131790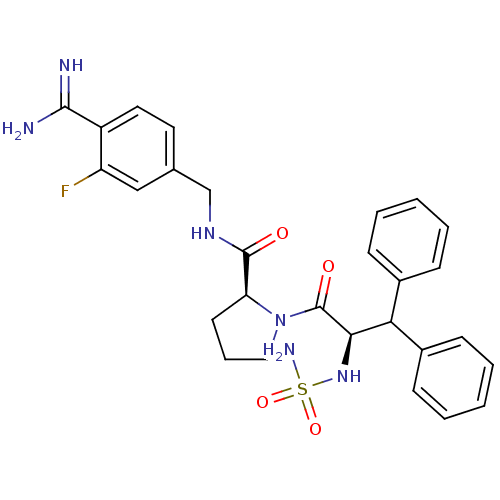 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131795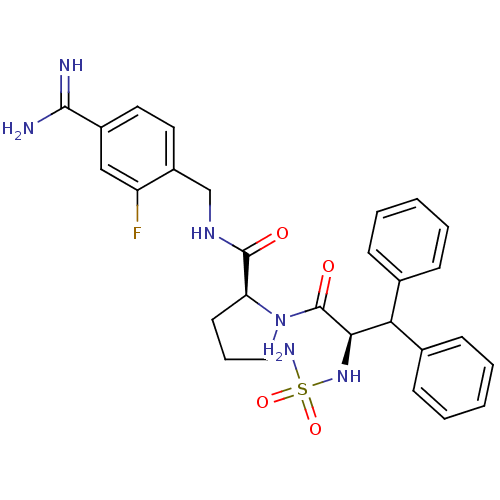 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131778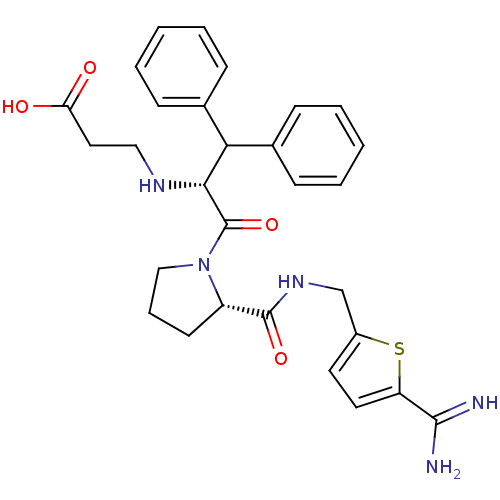 (3-(1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111120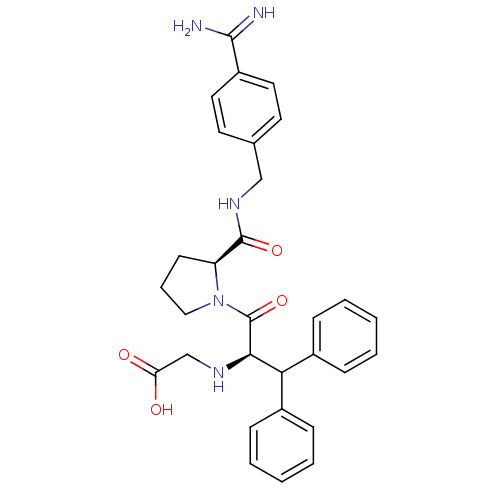 (2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111120 (2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131780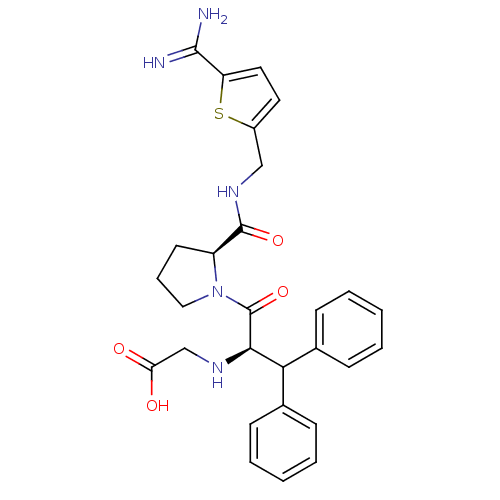 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131791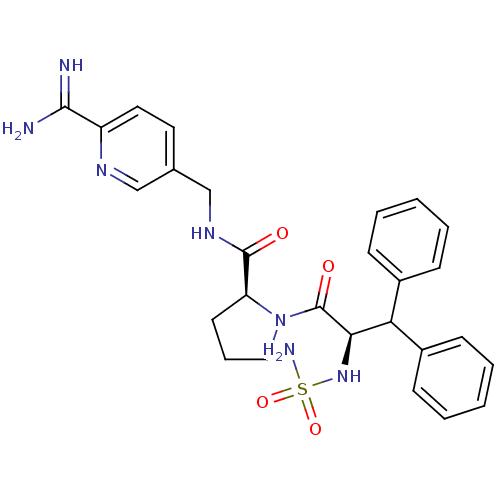 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131796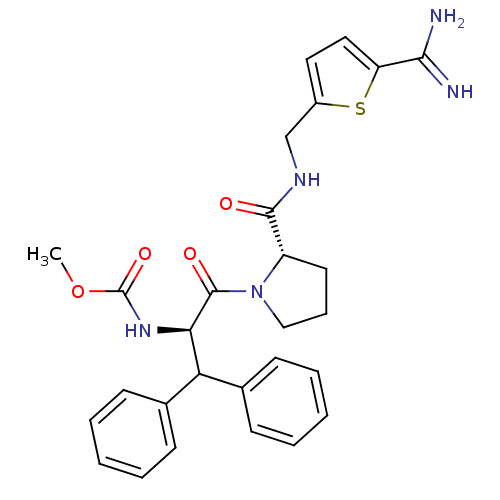 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131792 ((1-Benzhydryl-2-{2-[(6-carbamimidoyl-pyridin-3-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220995 (CHEMBL77788) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111102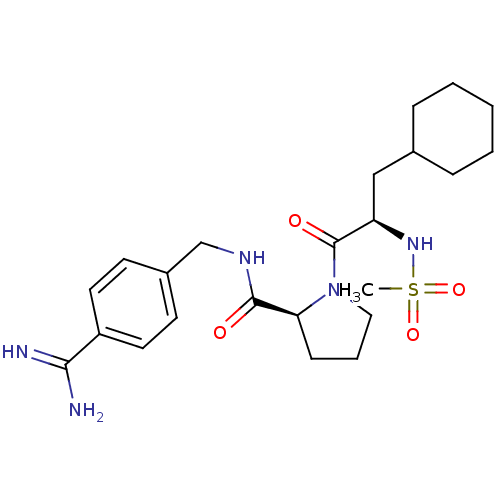 ((S)-1-((R)-3-Cyclohexyl-2-methanesulfonylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131798 (1-(2-Methylamino-3,3-diphenyl-propionyl)-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131782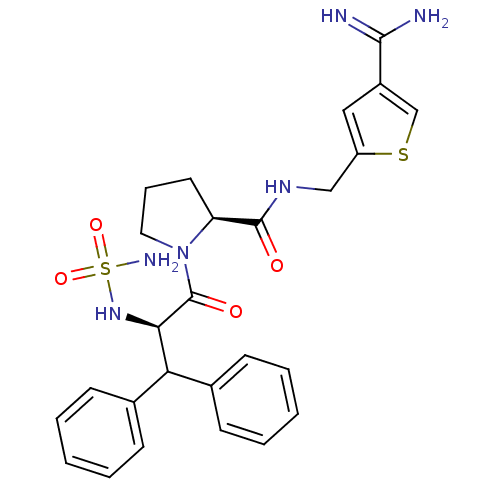 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111122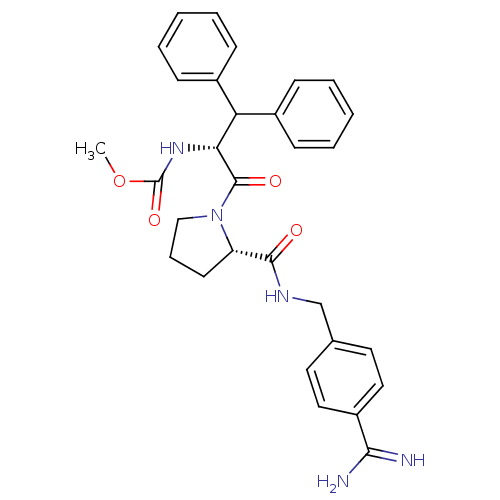 (2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111104 (2N-(4-Benzamidinemethyl)-1-[2-Amino-3,3-diphenyl-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111122 (2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221005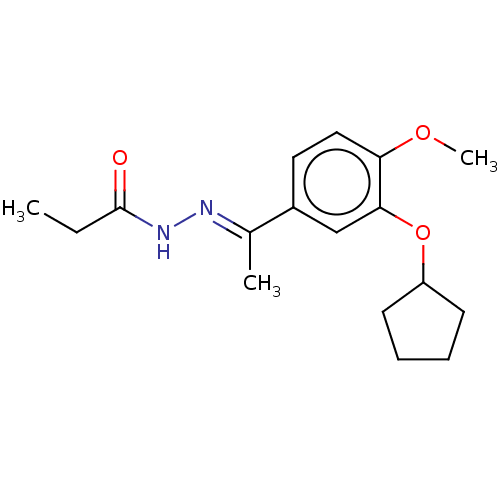 (CHEMBL75684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111109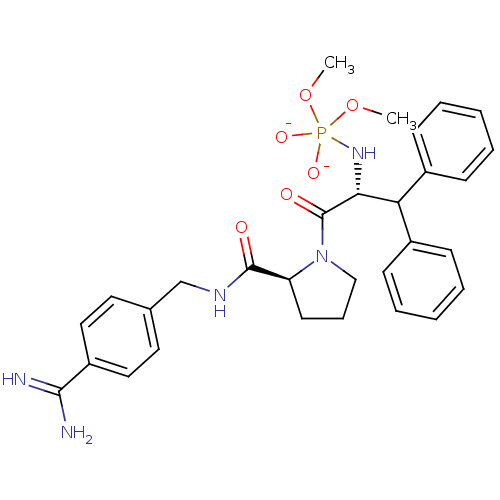 ({(R)-1-Benzhydryl-2-[(S)-2-(4-carbamimidoyl-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111098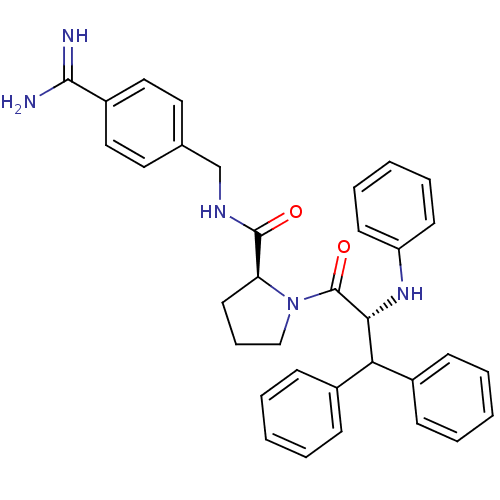 (2N-(4-Benzamidinemethyl)-1-[2-Phenylamino-3,3-diph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220998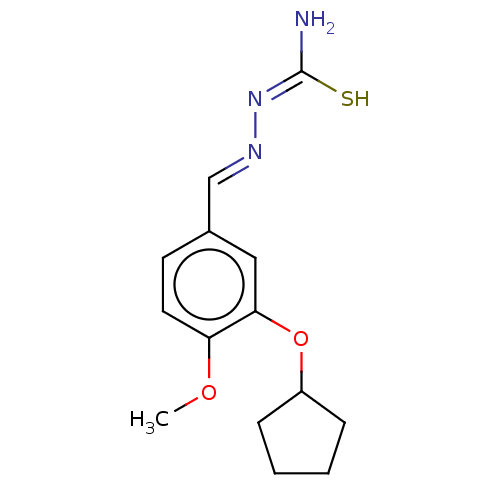 (CHEMBL76382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111103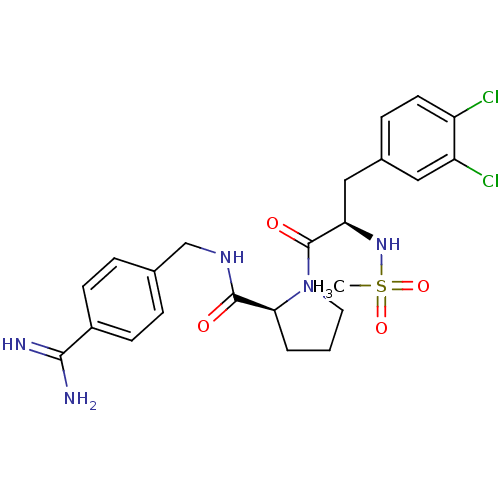 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-methanesulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080882 (Benzamidrazone analogue | CHEMBL312244) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221003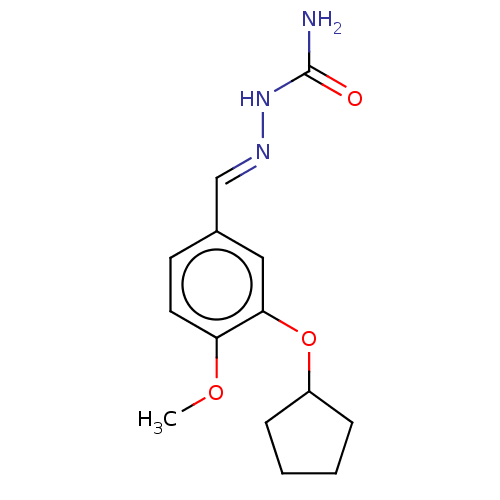 (CHEMBL432348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416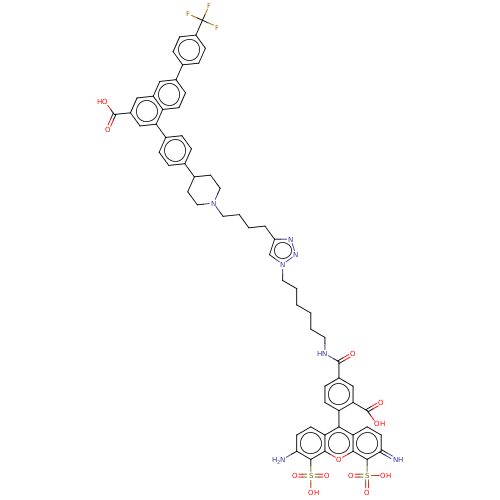 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of human P2Y14R | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220997 (CHEMBL78237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131787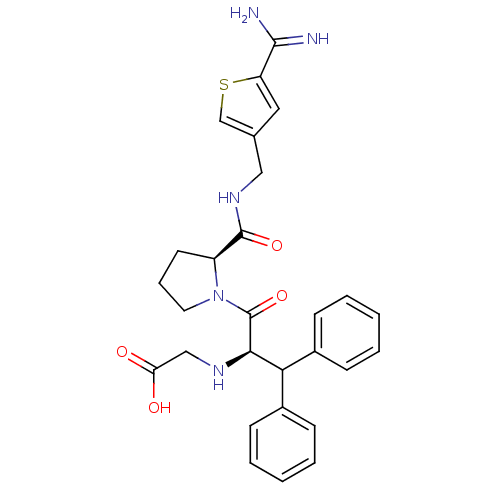 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284986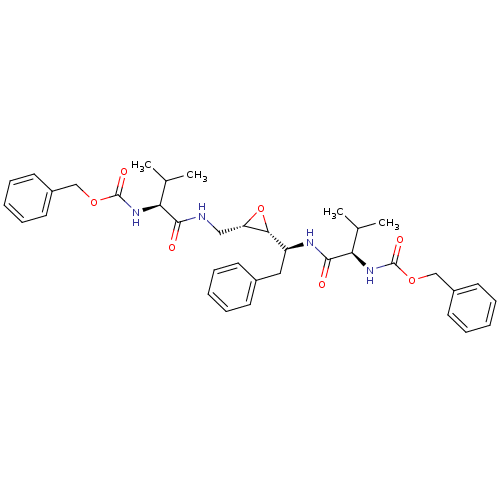 (CHEMBL55197 | [(R)-1-((S)-1-{(2R,3S)-3-[((S)-2-Ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111118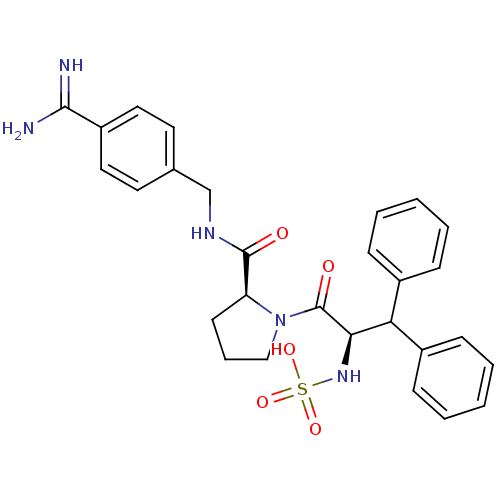 (2N-(4-Benzamidinemethyl)-1-[2-Hydroxysulfonamido-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080879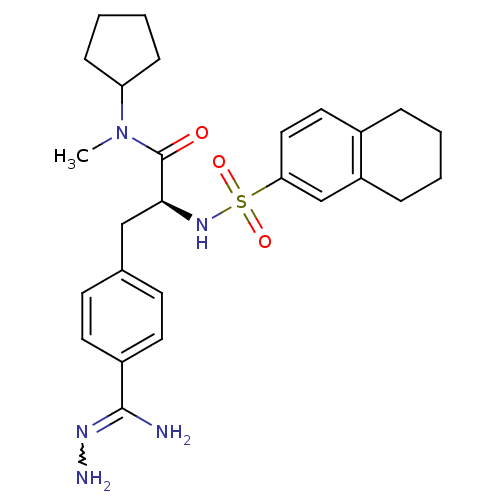 (Benzamidrazone analogue | CHEMBL82057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131781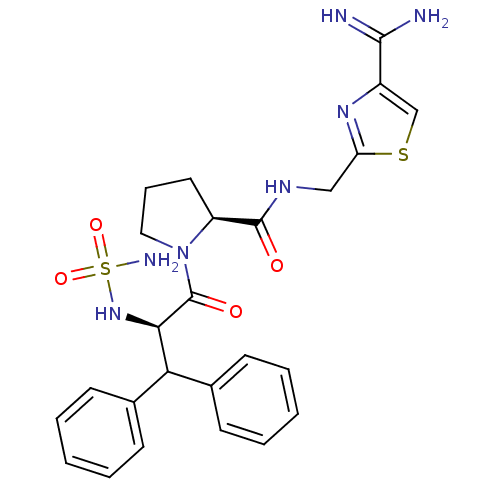 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM85177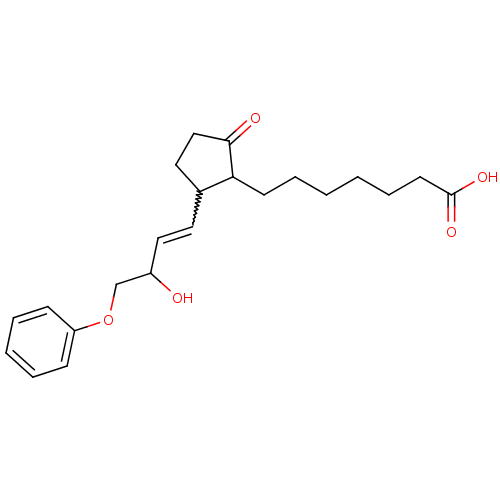 (CAS_80558-61-8 | M&B-28767 | NSC_119139) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by PDSP Ki Database | Biochim Biophys Acta 1483: 285-93 (2000) Article DOI: 10.1016/s1388-1981(99)00164-x BindingDB Entry DOI: 10.7270/Q2J964XQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221006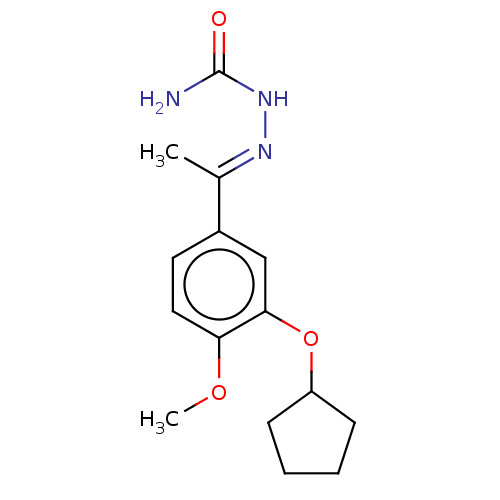 (CHEMBL77358) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111114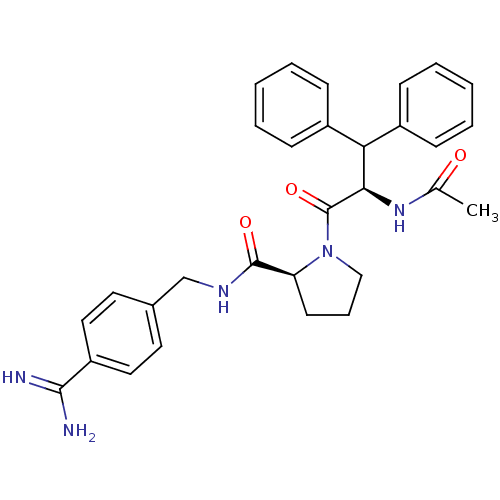 (2N-(4-Benzamidinemethyl)-1-[2-Acetylamino-3,3-diph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131797 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131779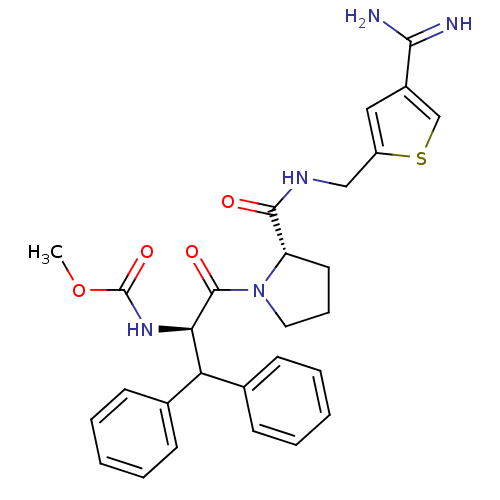 ((1-Benzhydryl-2-{2-[(4-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483880 (CHEMBL1774642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [3H]PIB from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080885 (Benzamidrazone analogue | CHEMBL79304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080883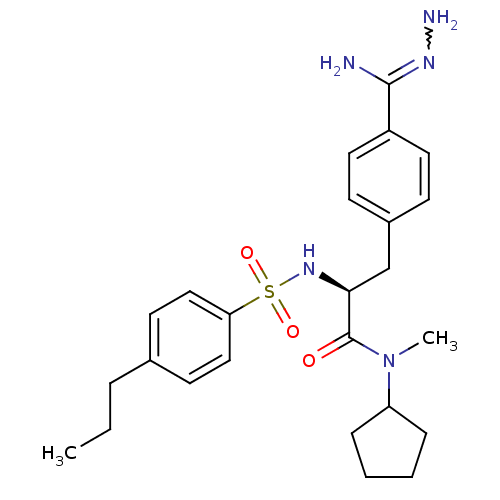 (Benzamidrazone analogue | CHEMBL310664) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111121 (2N-(4-Benzamidinemethyl)-1-[2-Cyclohexylaminosulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50252829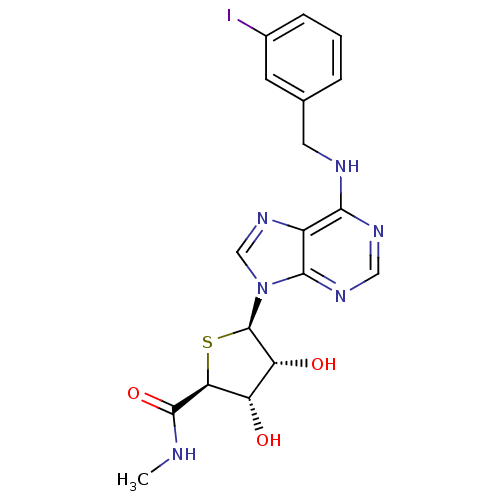 ((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7201 total ) | Next | Last >> |