| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C9 |
|---|
| Ligand | BDBM50263678 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_536101 (CHEMBL995047) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Jolidon, S; Alberati, D; Dowle, A; Fischer, H; Hainzl, D; Narquizian, R; Norcross, R; Pinard, E Design, synthesis and structure-activity relationship of simple bis-amides as potent inhibitors of GlyT1. Bioorg Med Chem Lett18:5533-6 (2008) [PubMed] Article Jolidon, S; Alberati, D; Dowle, A; Fischer, H; Hainzl, D; Narquizian, R; Norcross, R; Pinard, E Design, synthesis and structure-activity relationship of simple bis-amides as potent inhibitors of GlyT1. Bioorg Med Chem Lett18:5533-6 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C9 |
|---|
| Name: | Cytochrome P450 2C9 |
|---|
| Synonyms: | (R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55636.33 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11712 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKV
YGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKW
KEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICS
IIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFM
KSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTE
TTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYID
LLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFK
KSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVP
PFYQLCFIPV
|
|
|
|---|
| BDBM50263678 |
|---|
| n/a |
|---|
| Name | BDBM50263678 |
|---|
| Synonyms: | CHEMBL518287 | N-(2-((3-chlorophenyl)(phenyl)methylamino)-2-oxoethyl)-4-fluorobenzamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H18ClFN2O2 |
|---|
| Mol. Mass. | 396.842 |
|---|
| SMILES | Fc1ccc(cc1)C(=O)NCC(=O)NC(c1ccccc1)c1cccc(Cl)c1 |
|---|
| Structure | 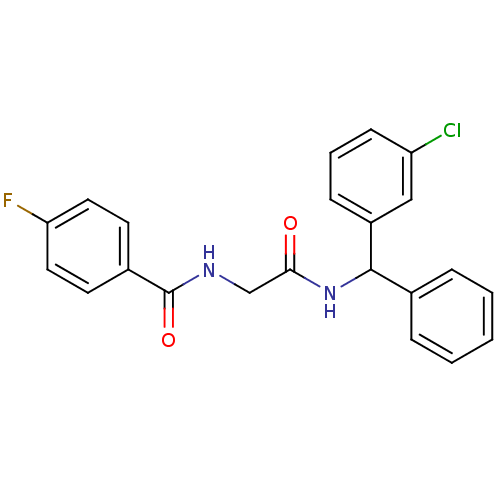
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jolidon, S; Alberati, D; Dowle, A; Fischer, H; Hainzl, D; Narquizian, R; Norcross, R; Pinard, E Design, synthesis and structure-activity relationship of simple bis-amides as potent inhibitors of GlyT1. Bioorg Med Chem Lett18:5533-6 (2008) [PubMed] Article
Jolidon, S; Alberati, D; Dowle, A; Fischer, H; Hainzl, D; Narquizian, R; Norcross, R; Pinard, E Design, synthesis and structure-activity relationship of simple bis-amides as potent inhibitors of GlyT1. Bioorg Med Chem Lett18:5533-6 (2008) [PubMed] Article