 Found 838 hits with Last Name = 'jolidon' and Initial = 's'
Found 838 hits with Last Name = 'jolidon' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130785
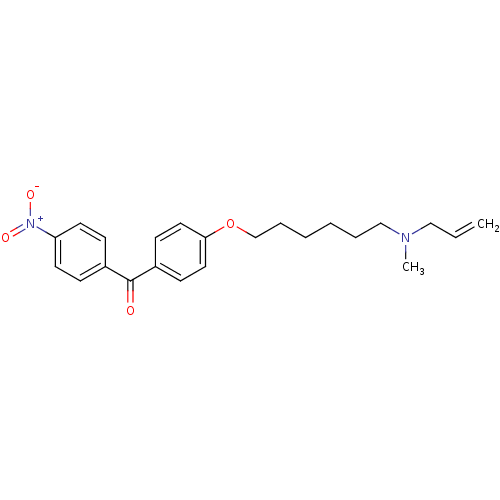
(CHEMBL542453 | CHEMBL609978 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(cc1)[N+]([O-])=O)CC=C Show InChI InChI=1S/C23H28N2O4/c1-3-16-24(2)17-6-4-5-7-18-29-22-14-10-20(11-15-22)23(26)19-8-12-21(13-9-19)25(27)28/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128054
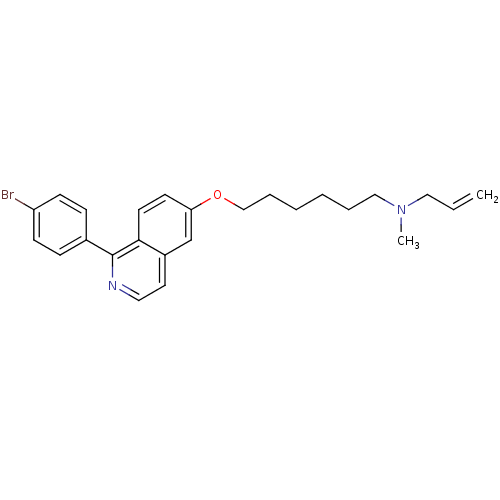
(Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...)Show SMILES CN(CCCCCCOc1ccc2c(nccc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C25H29BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-15,19H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128055
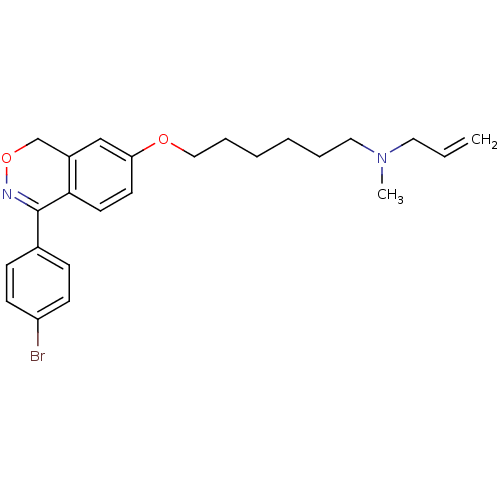
(Allyl-{6-[4-(4-bromo-phenyl)-1H-benzo[d][1,2]oxazi...)Show SMILES CN(CCCCCCOc1ccc2C(=NOCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C24H29BrN2O2/c1-3-14-27(2)15-6-4-5-7-16-28-22-12-13-23-20(17-22)18-29-26-24(23)19-8-10-21(25)11-9-19/h3,8-13,17H,1,4-7,14-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130788
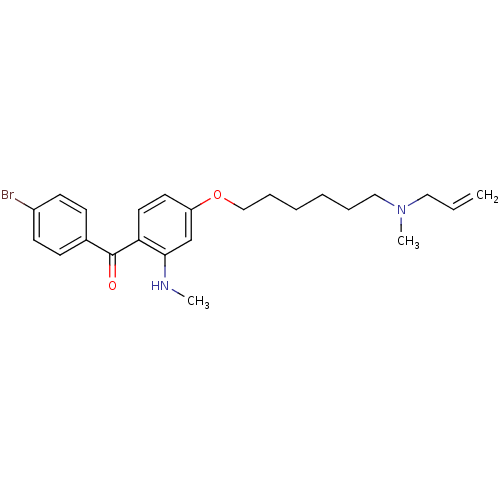
(CHEMBL116705 | CHEMBL611484 | {4-[6-(Allyl-methyl-...)Show SMILES CNc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H31BrN2O2/c1-4-15-27(3)16-7-5-6-8-17-29-21-13-14-22(23(18-21)26-2)24(28)19-9-11-20(25)12-10-19/h4,9-14,18,26H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130783

(CHEMBL115020 | CHEMBL611485 | {4-[6-(Allyl-methyl-...)Show SMILES COc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO3/c1-4-15-26(2)16-7-5-6-8-17-29-21-13-14-22(23(18-21)28-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128050
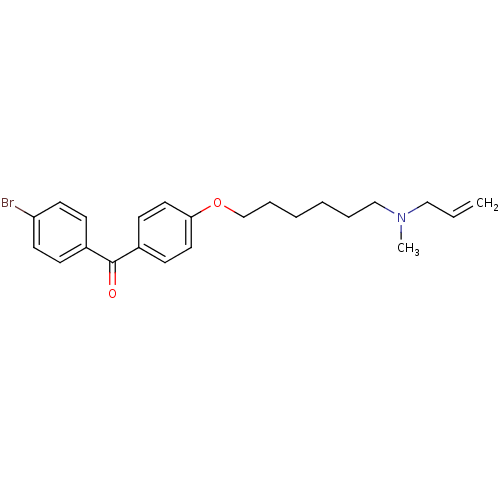
(CHEMBL114259 | CHEMBL416694 | N-allyl-6-(4-(4-brom...)Show InChI InChI=1S/C23H28BrNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130789

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[b]thiophen-6-yl...)Show SMILES CN(CCCCCCOc1ccc2c(csc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H28BrNOS/c1-3-14-26(2)15-6-4-5-7-16-27-21-12-13-22-23(18-28-24(22)17-21)19-8-10-20(25)11-9-19/h3,8-13,17-18H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130786
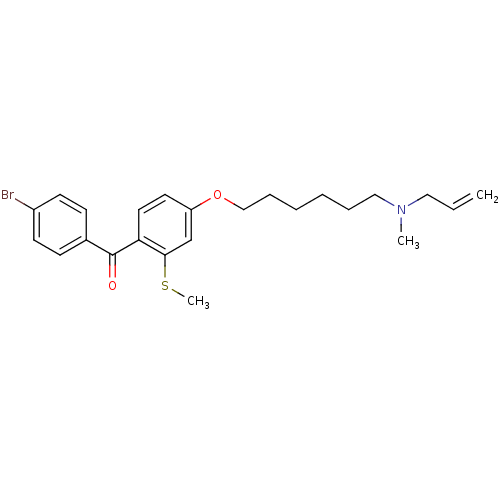
(CHEMBL115923 | CHEMBL611486 | {4-[6-(Allyl-methyl-...)Show SMILES CSc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO2S/c1-4-15-26(2)16-7-5-6-8-17-28-21-13-14-22(23(18-21)29-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130774
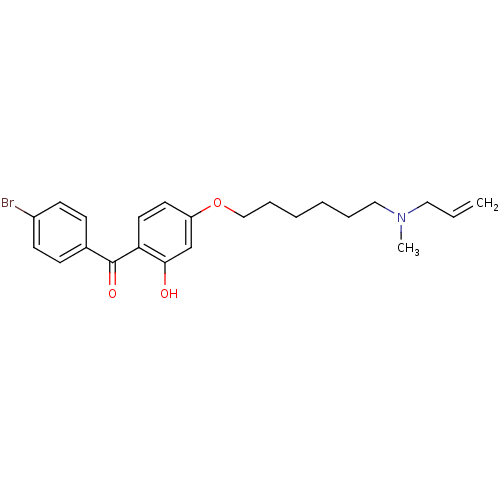
(CHEMBL115375 | CHEMBL611757 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(O)c1)CC=C Show InChI InChI=1S/C23H28BrNO3/c1-3-14-25(2)15-6-4-5-7-16-28-20-12-13-21(22(26)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17,26H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130775
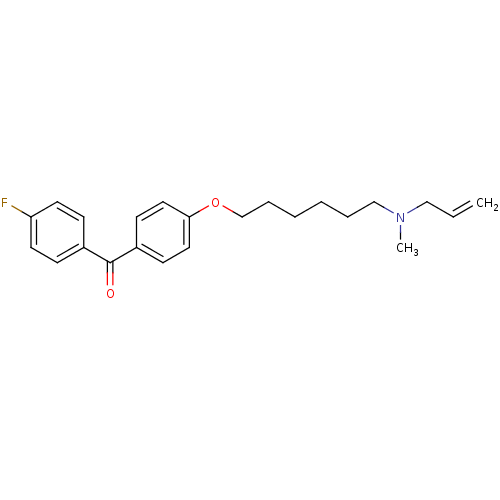
(CHEMBL554209 | CHEMBL611758 | {4-[6-(Allyl-methyl-...)Show InChI InChI=1S/C23H28FNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130772
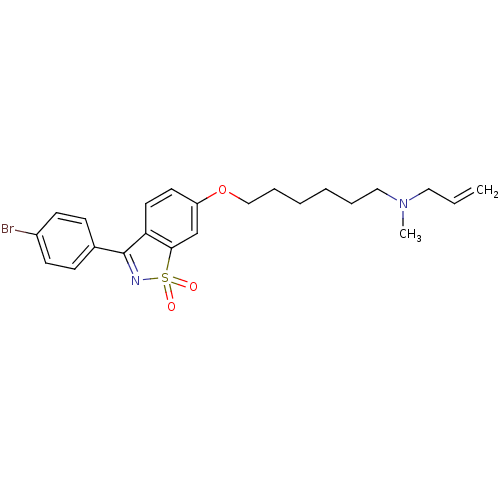
(Allyl-{6-[3-(4-bromo-phenyl)-1,1-dioxo-1H-1lambda*...)Show SMILES CN(CCCCCCOc1ccc2C(=NS(=O)(=O)c2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C23H27BrN2O3S/c1-3-14-26(2)15-6-4-5-7-16-29-20-12-13-21-22(17-20)30(27,28)25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130784

(Allyl-{6-[1-(4-bromo-phenyl)-3,4-dihydro-isoquinol...)Show SMILES CN(CCCCCCOc1ccc2C(=NCCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C25H31BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-13,19H,1,4-7,14-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130787
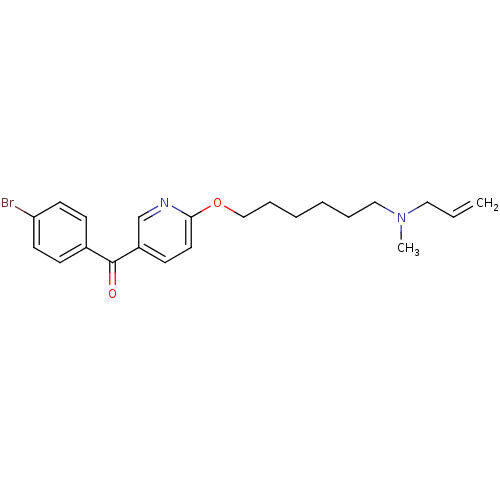
(CHEMBL114266 | CHEMBL612025 | {6-[6-(Allyl-methyl-...)Show InChI InChI=1S/C22H27BrN2O2/c1-3-14-25(2)15-6-4-5-7-16-27-21-13-10-19(17-24-21)22(26)18-8-11-20(23)12-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130776

(Allyl-{6-[4-(4-bromo-phenyl)-2H-chromen-7-yloxy]-h...)Show SMILES CN(CCCCCCOc1ccc2C(=CCOc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C25H30BrNO2/c1-3-15-27(2)16-6-4-5-7-17-28-22-12-13-24-23(14-18-29-25(24)19-22)20-8-10-21(26)11-9-20/h3,8-14,19H,1,4-7,15-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130781
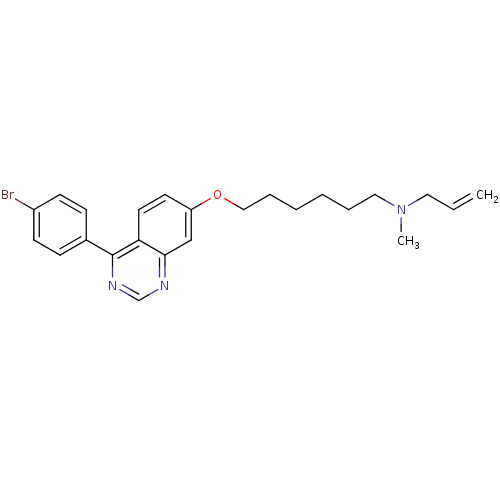
(Allyl-{6-[4-(4-bromo-phenyl)-quinazolin-7-yloxy]-h...)Show SMILES CN(CCCCCCOc1ccc2c(ncnc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H28BrN3O/c1-3-14-28(2)15-6-4-5-7-16-29-21-12-13-22-23(17-21)26-18-27-24(22)19-8-10-20(25)11-9-19/h3,8-13,17-18H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188855

(1-phenyl-8-(1-phenyl-cyclohexyl)-1,3,8-triaza-spir...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC1)C1(CCCCC1)c1ccccc1 Show InChI InChI=1S/C25H31N3O/c29-23-25(28(20-26-23)22-12-6-2-7-13-22)16-18-27(19-17-25)24(14-8-3-9-15-24)21-10-4-1-5-11-21/h1-2,4-7,10-13H,3,8-9,14-20H2,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50188855

(1-phenyl-8-(1-phenyl-cyclohexyl)-1,3,8-triaza-spir...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC1)C1(CCCCC1)c1ccccc1 Show InChI InChI=1S/C25H31N3O/c29-23-25(28(20-26-23)22-12-6-2-7-13-22)16-18-27(19-17-25)24(14-8-3-9-15-24)21-10-4-1-5-11-21/h1-2,4-7,10-13H,3,8-9,14-20H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]NOP from human NOP receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130777
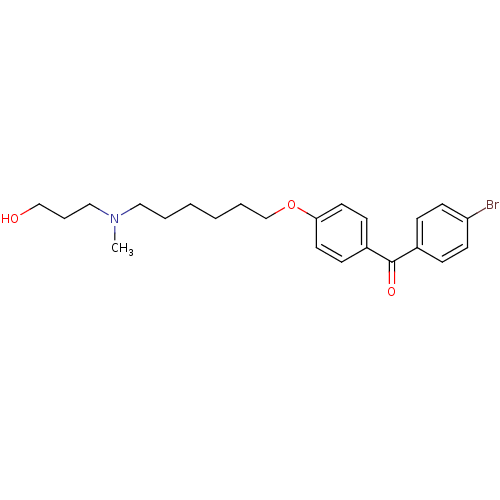
((4-Bromo-phenyl)-(4-{6-[(3-hydroxy-propyl)-methyl-...)Show InChI InChI=1S/C23H30BrNO3/c1-25(16-6-17-26)15-4-2-3-5-18-28-22-13-9-20(10-14-22)23(27)19-7-11-21(24)12-8-19/h7-14,26H,2-6,15-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128058
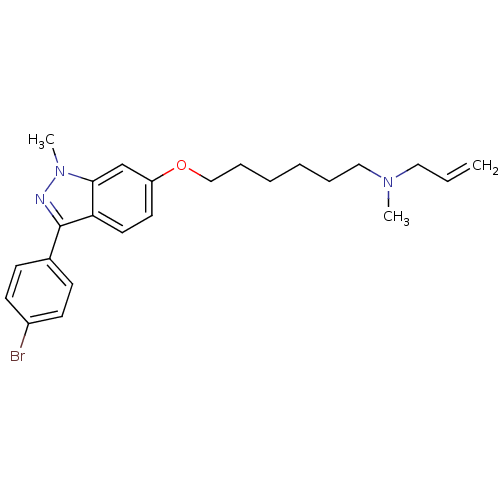
(ALLYL-{6-[3-(4-BROMO-PHENYL)-1-METHYL-1H-INDAZOL-6...)Show SMILES CN(CCCCCCOc1ccc2c(nn(C)c2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H30BrN3O/c1-4-15-27(2)16-7-5-6-8-17-29-21-13-14-22-23(18-21)28(3)26-24(22)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130779
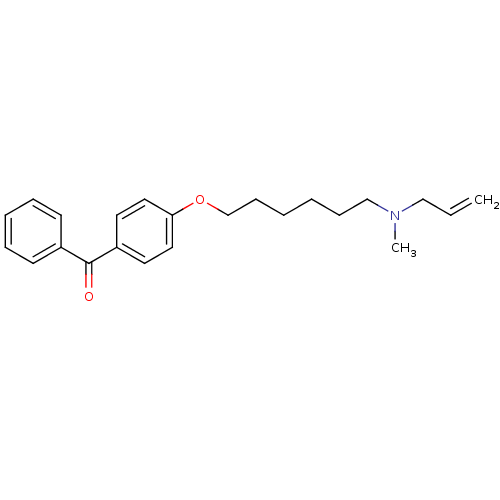
(CHEMBL553281 | CHEMBL611755 | {4-[6-(Allyl-methyl-...)Show InChI InChI=1S/C23H29NO2/c1-3-17-24(2)18-9-4-5-10-19-26-22-15-13-21(14-16-22)23(25)20-11-7-6-8-12-20/h3,6-8,11-16H,1,4-5,9-10,17-19H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130771
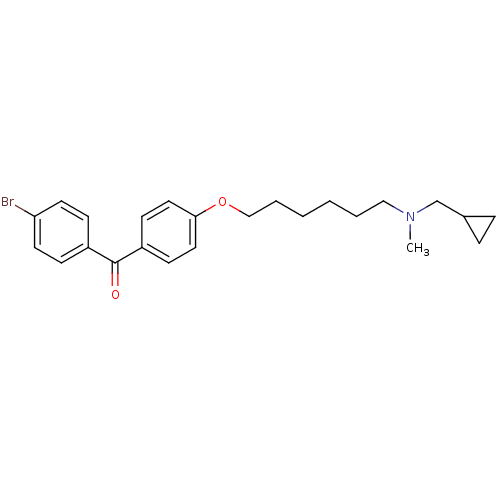
((4-Bromo-phenyl)-{4-[6-(cyclopropylmethyl-methyl-a...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(Br)cc1)CC1CC1 Show InChI InChI=1S/C24H30BrNO2/c1-26(18-19-6-7-19)16-4-2-3-5-17-28-23-14-10-21(11-15-23)24(27)20-8-12-22(25)13-9-20/h8-15,19H,2-7,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130778
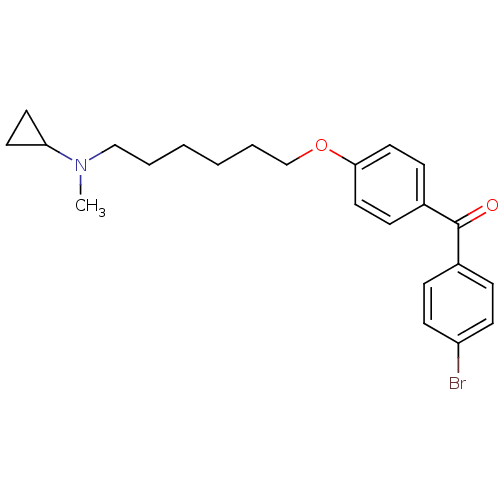
((4-Bromo-phenyl)-{4-[6-(cyclopropyl-methyl-amino)-...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(Br)cc1)C1CC1 Show InChI InChI=1S/C23H28BrNO2/c1-25(21-12-13-21)16-4-2-3-5-17-27-22-14-8-19(9-15-22)23(26)18-6-10-20(24)11-7-18/h6-11,14-15,21H,2-5,12-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35.3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50175903
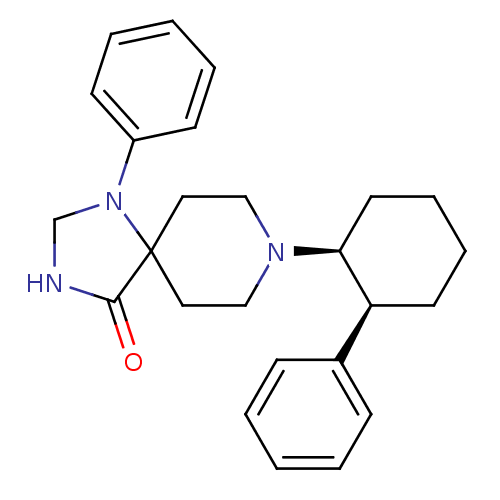
(1-Phenyl-8-((cis,1S,2S)-2-phenyl-cyclohexyl)-1,3,8...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC1)[C@H]1CCCC[C@H]1c1ccccc1 Show InChI InChI=1S/C25H31N3O/c29-24-25(28(19-26-24)21-11-5-2-6-12-21)15-17-27(18-16-25)23-14-8-7-13-22(23)20-9-3-1-4-10-20/h1-6,9-12,22-23H,7-8,13-19H2,(H,26,29)/t22-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4305-10 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.064
BindingDB Entry DOI: 10.7270/Q28C9VVV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188374
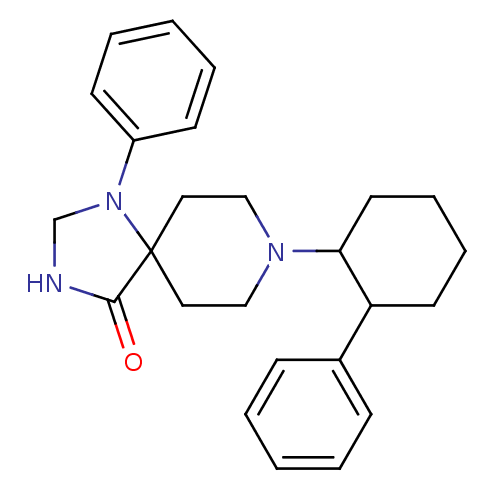
(1-phenyl-8-(2-phenyl-cyclohexyl)-1,3,8-triaza-spir...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC1)C1CCCCC1c1ccccc1 Show InChI InChI=1S/C25H31N3O/c29-24-25(28(19-26-24)21-11-5-2-6-12-21)15-17-27(18-16-25)23-14-8-7-13-22(23)20-9-3-1-4-10-20/h1-6,9-12,22-23H,7-8,13-19H2,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4311-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.058
BindingDB Entry DOI: 10.7270/Q2Z31Z8W |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent glycine transporter 1
(Homo sapiens (Human)) | BDBM50263360
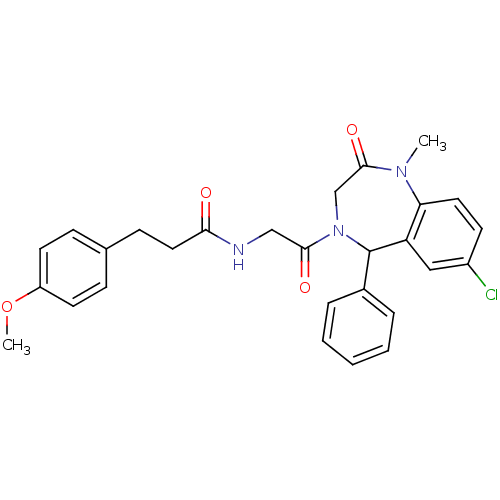
(CHEMBL476555 | N-(2-(7-chloro-1-methyl-2-oxo-5-phe...)Show SMILES COc1ccc(CCC(=O)NCC(=O)N2CC(=O)N(C)c3ccc(Cl)cc3C2c2ccccc2)cc1 Show InChI InChI=1S/C28H28ClN3O4/c1-31-24-14-11-21(29)16-23(24)28(20-6-4-3-5-7-20)32(18-27(31)35)26(34)17-30-25(33)15-10-19-8-12-22(36-2)13-9-19/h3-9,11-14,16,28H,10,15,17-18H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human glycine transporter 1 |
Bioorg Med Chem Lett 18: 5533-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.005
BindingDB Entry DOI: 10.7270/Q2BR8S19 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50356909
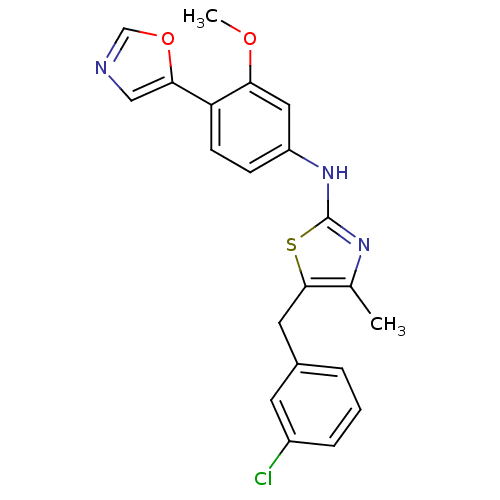
(CHEMBL1915558)Show SMILES COc1cc(Nc2nc(C)c(Cc3cccc(Cl)c3)s2)ccc1-c1cnco1 Show InChI InChI=1S/C21H18ClN3O2S/c1-13-20(9-14-4-3-5-15(22)8-14)28-21(24-13)25-16-6-7-17(18(10-16)26-2)19-11-23-12-27-19/h3-8,10-12H,9H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of IMPDH2 |
Bioorg Med Chem Lett 21: 6554-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.060
BindingDB Entry DOI: 10.7270/Q2QZ2BCM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50265098
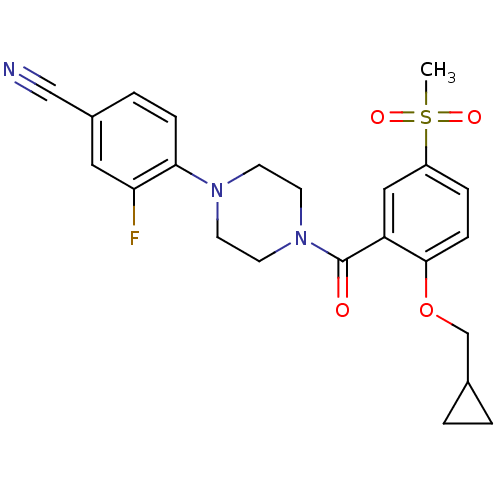
(4-(4-(2-(cyclopropylmethoxy)-5-(methylsulfonyl)ben...)Show SMILES CS(=O)(=O)c1ccc(OCC2CC2)c(c1)C(=O)N1CCN(CC1)c1ccc(cc1F)C#N Show InChI InChI=1S/C23H24FN3O4S/c1-32(29,30)18-5-7-22(31-15-16-2-3-16)19(13-18)23(28)27-10-8-26(9-11-27)21-6-4-17(14-25)12-20(21)24/h4-7,12-13,16H,2-3,8-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ERG potassium channel by patch-clamp assay |
Bioorg Med Chem Lett 18: 5134-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.086
BindingDB Entry DOI: 10.7270/Q28915PM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50265098

(4-(4-(2-(cyclopropylmethoxy)-5-(methylsulfonyl)ben...)Show SMILES CS(=O)(=O)c1ccc(OCC2CC2)c(c1)C(=O)N1CCN(CC1)c1ccc(cc1F)C#N Show InChI InChI=1S/C23H24FN3O4S/c1-32(29,30)18-5-7-22(31-15-16-2-3-16)19(13-18)23(28)27-10-8-26(9-11-27)21-6-4-17(14-25)12-20(21)24/h4-7,12-13,16H,2-3,8-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch clamp assay |
J Med Chem 53: 4603-14 (2010)
Article DOI: 10.1021/jm100210p
BindingDB Entry DOI: 10.7270/Q28916TT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50322424

(3-Fluoro-4-[4-(2-isobutoxy-5-methanesulfonyl-benzo...)Show SMILES CC(C)COc1ccc(cc1C(=O)N1CCN(CC1)c1ccc(cc1F)C#N)S(C)(=O)=O Show InChI InChI=1S/C23H26FN3O4S/c1-16(2)15-31-22-7-5-18(32(3,29)30)13-19(22)23(28)27-10-8-26(9-11-27)21-6-4-17(14-25)12-20(21)24/h4-7,12-13,16H,8-11,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch clamp assay |
J Med Chem 53: 4603-14 (2010)
Article DOI: 10.1021/jm100210p
BindingDB Entry DOI: 10.7270/Q28916TT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188806
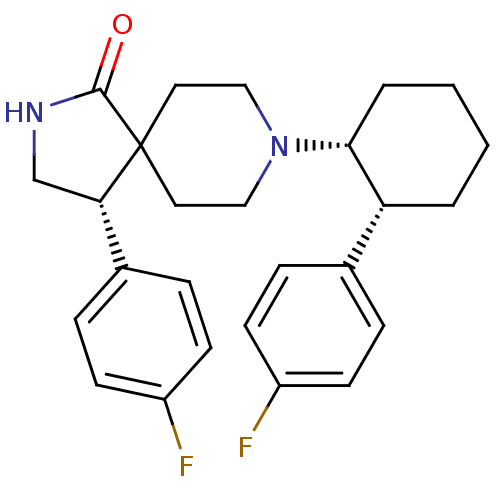
((S)-4-(4-fluoro-phenyl)-8-[(1R,2R)-2-(4-fluoro-phe...)Show SMILES Fc1ccc(cc1)[C@@H]1CNC(=O)C11CCN(CC1)[C@@H]1CCCC[C@@H]1c1ccc(F)cc1 Show InChI InChI=1S/C26H30F2N2O/c27-20-9-5-18(6-10-20)22-3-1-2-4-24(22)30-15-13-26(14-16-30)23(17-29-25(26)31)19-7-11-21(28)12-8-19/h5-12,22-24H,1-4,13-17H2,(H,29,31)/t22-,23+,24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4305-10 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.064
BindingDB Entry DOI: 10.7270/Q28C9VVV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50356910
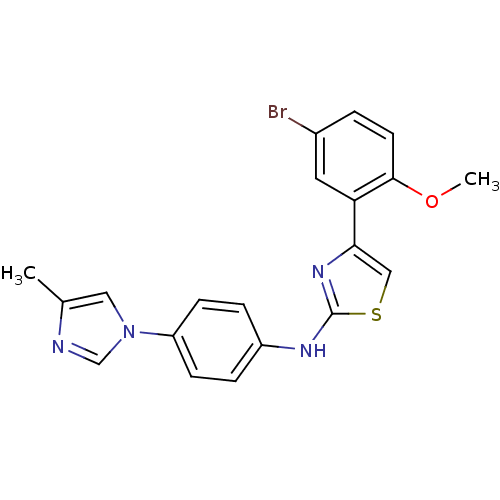
(CHEMBL1915565)Show SMILES COc1ccc(Br)cc1-c1csc(Nc2ccc(cc2)-n2cnc(C)c2)n1 Show InChI InChI=1S/C20H17BrN4OS/c1-13-10-25(12-22-13)16-6-4-15(5-7-16)23-20-24-18(11-27-20)17-9-14(21)3-8-19(17)26-2/h3-12H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 6554-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.060
BindingDB Entry DOI: 10.7270/Q2QZ2BCM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50322428
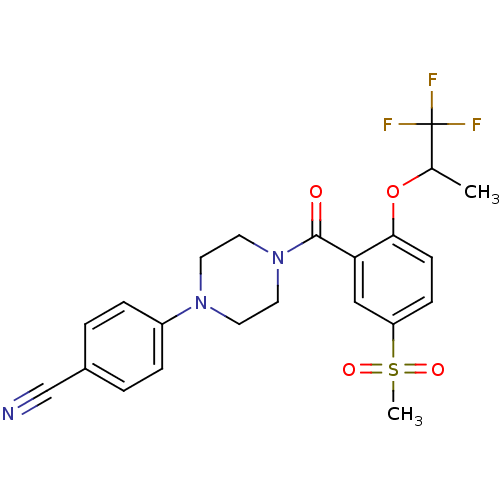
(CHEMBL1171498 | rac-4-{4-[5-Methanesulfonyl-2-(2,2...)Show SMILES CC(Oc1ccc(cc1C(=O)N1CCN(CC1)c1ccc(cc1)C#N)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C22H22F3N3O4S/c1-15(22(23,24)25)32-20-8-7-18(33(2,30)31)13-19(20)21(29)28-11-9-27(10-12-28)17-5-3-16(14-26)4-6-17/h3-8,13,15H,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch clamp assay |
J Med Chem 53: 4603-14 (2010)
Article DOI: 10.1021/jm100210p
BindingDB Entry DOI: 10.7270/Q28916TT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50188849
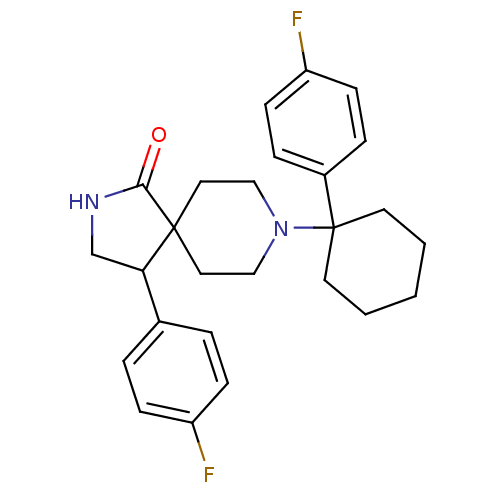
(4-(4-fluoro-phenyl)-8-[1-(4-fluoro-phenyl)-cyclohe...)Show SMILES Fc1ccc(cc1)C1CNC(=O)C11CCN(CC1)C1(CCCCC1)c1ccc(F)cc1 Show InChI InChI=1S/C26H30F2N2O/c27-21-8-4-19(5-9-21)23-18-29-24(31)25(23)14-16-30(17-15-25)26(12-2-1-3-13-26)20-6-10-22(28)11-7-20/h4-11,23H,1-3,12-18H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of hERG potassium channel expressed in CHO cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
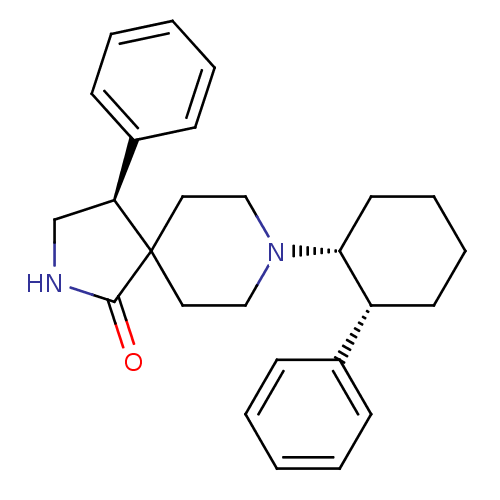
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50188803
((S)-4-phenyl-8-((1R,2R)-2-phenyl-cyclohexyl)-2,8-d...)Show SMILES O=C1NC[C@@H](c2ccccc2)C11CCN(CC1)[C@@H]1CCCC[C@@H]1c1ccccc1 Show InChI InChI=1S/C26H32N2O/c29-25-26(23(19-27-25)21-11-5-2-6-12-21)15-17-28(18-16-26)24-14-8-7-13-22(24)20-9-3-1-4-10-20/h1-6,9-12,22-24H,7-8,13-19H2,(H,27,29)/t22-,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of hERG potassium channel expressed in CHO cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 4311-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.058
BindingDB Entry DOI: 10.7270/Q2Z31Z8W |
More data for this
Ligand-Target Pair | |
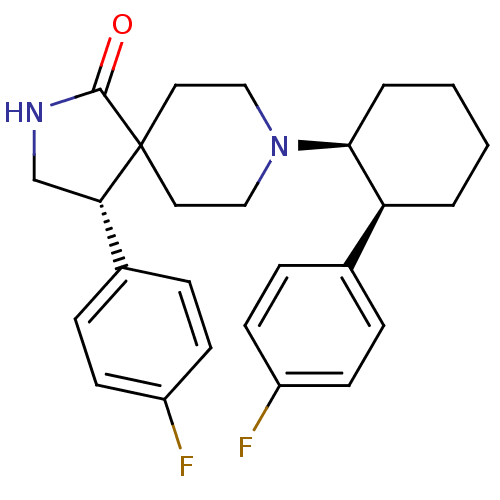
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188813
((S)-4-(4-fluoro-phenyl)-8-[(1S,2S)-2-(4-fluoro-phe...)Show SMILES Fc1ccc(cc1)[C@@H]1CNC(=O)C11CCN(CC1)[C@H]1CCCC[C@H]1c1ccc(F)cc1 Show InChI InChI=1S/C26H30F2N2O/c27-20-9-5-18(6-10-20)22-3-1-2-4-24(22)30-15-13-26(14-16-30)23(17-29-25(26)31)19-7-11-21(28)12-8-19/h5-12,22-24H,1-4,13-17H2,(H,29,31)/t22-,23-,24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4305-10 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.064
BindingDB Entry DOI: 10.7270/Q28C9VVV |
More data for this
Ligand-Target Pair | |
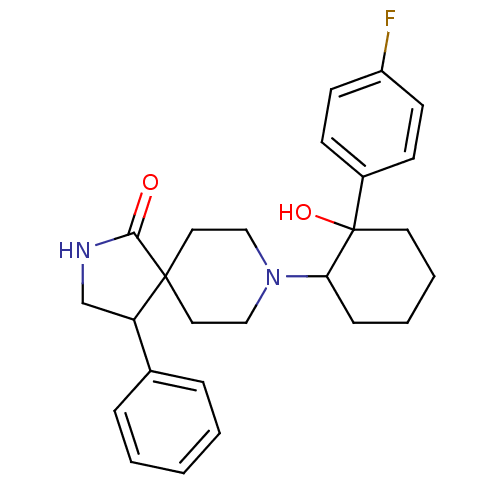
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50188364
(8-[2-(4-fluoro-phenyl)-2-hydroxy-cyclohexyl]-4-phe...)Show SMILES OC1(CCCCC1N1CCC2(CC1)C(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C26H31FN2O2/c27-21-11-9-20(10-12-21)26(31)13-5-4-8-23(26)29-16-14-25(15-17-29)22(18-28-24(25)30)19-6-2-1-3-7-19/h1-3,6-7,9-12,22-23,31H,4-5,8,13-18H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of hERG potassium channel expressed in CHO cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 4311-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.058
BindingDB Entry DOI: 10.7270/Q2Z31Z8W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50322426
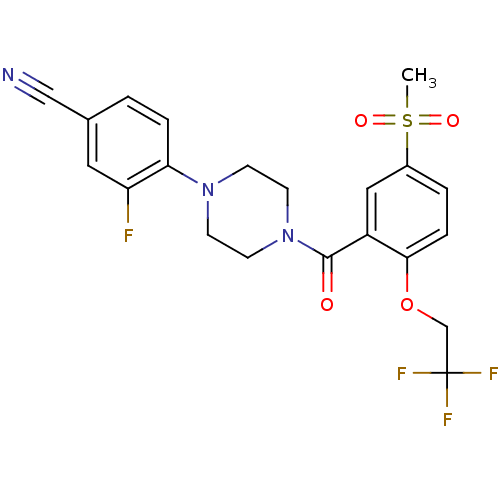
(3-Fluoro-4-{4-[5-methanesulfonyl-2-(2,2,2-trifluor...)Show SMILES CS(=O)(=O)c1ccc(OCC(F)(F)F)c(c1)C(=O)N1CCN(CC1)c1ccc(cc1F)C#N Show InChI InChI=1S/C21H19F4N3O4S/c1-33(30,31)15-3-5-19(32-13-21(23,24)25)16(11-15)20(29)28-8-6-27(7-9-28)18-4-2-14(12-26)10-17(18)22/h2-5,10-11H,6-9,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch clamp assay |
J Med Chem 53: 4603-14 (2010)
Article DOI: 10.1021/jm100210p
BindingDB Entry DOI: 10.7270/Q28916TT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50356911

(CHEMBL1915566)Show SMILES COc1ccc(Br)cc1-c1csc(Nc2ccc(c(OC)c2)-n2cnc(C)c2)n1 Show InChI InChI=1S/C21H19BrN4O2S/c1-13-10-26(12-23-13)18-6-5-15(9-20(18)28-3)24-21-25-17(11-29-21)16-8-14(22)4-7-19(16)27-2/h4-12H,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 6554-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.060
BindingDB Entry DOI: 10.7270/Q2QZ2BCM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188376
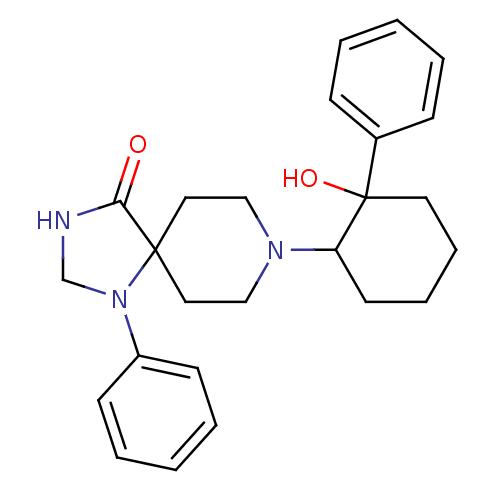
(8-(2-hydroxy-2-phenyl-cyclohexyl)-1-phenyl-1,3,8-t...)Show SMILES OC1(CCCCC1N1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H31N3O2/c29-23-24(28(19-26-23)21-11-5-2-6-12-21)15-17-27(18-16-24)22-13-7-8-14-25(22,30)20-9-3-1-4-10-20/h1-6,9-12,22,30H,7-8,13-19H2,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4311-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.058
BindingDB Entry DOI: 10.7270/Q2Z31Z8W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188843
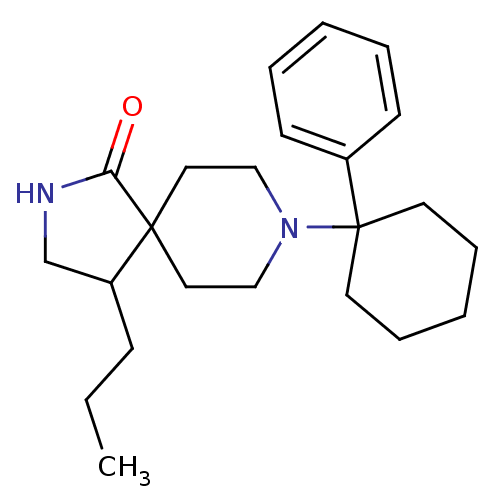
(8-(1-phenyl-cyclohexyl)-4-propyl-2,8-diaza-spiro[4...)Show InChI InChI=1S/C23H34N2O/c1-2-9-20-18-24-21(26)22(20)14-16-25(17-15-22)23(12-7-4-8-13-23)19-10-5-3-6-11-19/h3,5-6,10-11,20H,2,4,7-9,12-18H2,1H3,(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188843

(8-(1-phenyl-cyclohexyl)-4-propyl-2,8-diaza-spiro[4...)Show InChI InChI=1S/C23H34N2O/c1-2-9-20-18-24-21(26)22(20)14-16-25(17-15-22)23(12-7-4-8-13-23)19-10-5-3-6-11-19/h3,5-6,10-11,20H,2,4,7-9,12-18H2,1H3,(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188843

(8-(1-phenyl-cyclohexyl)-4-propyl-2,8-diaza-spiro[4...)Show InChI InChI=1S/C23H34N2O/c1-2-9-20-18-24-21(26)22(20)14-16-25(17-15-22)23(12-7-4-8-13-23)19-10-5-3-6-11-19/h3,5-6,10-11,20H,2,4,7-9,12-18H2,1H3,(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50322427
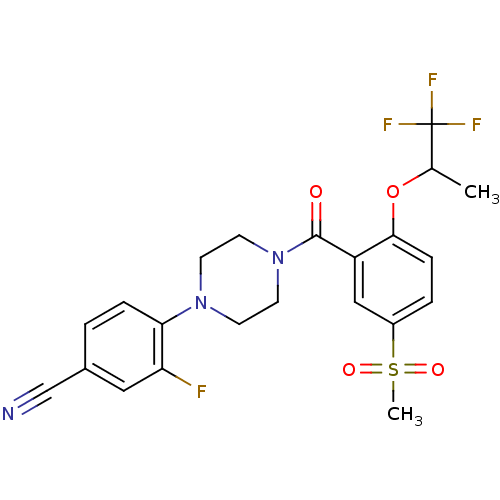
(CHEMBL1172999 | rac-Fluoro-4-{4-[5-methanesulfonyl...)Show SMILES CC(Oc1ccc(cc1C(=O)N1CCN(CC1)c1ccc(cc1F)C#N)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C22H21F4N3O4S/c1-14(22(24,25)26)33-20-6-4-16(34(2,31)32)12-17(20)21(30)29-9-7-28(8-10-29)19-5-3-15(13-27)11-18(19)23/h3-6,11-12,14H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch clamp assay |
J Med Chem 53: 4603-14 (2010)
Article DOI: 10.1021/jm100210p
BindingDB Entry DOI: 10.7270/Q28916TT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188879
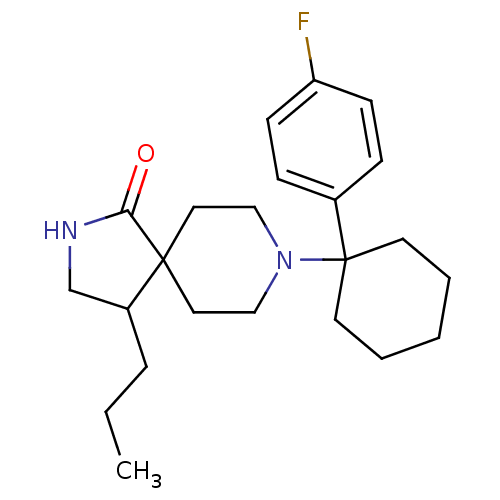
(CHEMBL214057 | rac-8-[1-(4-fluoro-phenyl)-cyclohex...)Show SMILES CCCC1CNC(=O)C11CCN(CC1)C1(CCCCC1)c1ccc(F)cc1 Show InChI InChI=1S/C23H33FN2O/c1-2-6-19-17-25-21(27)22(19)13-15-26(16-14-22)23(11-4-3-5-12-23)18-7-9-20(24)10-8-18/h7-10,19H,2-6,11-17H2,1H3,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50322425
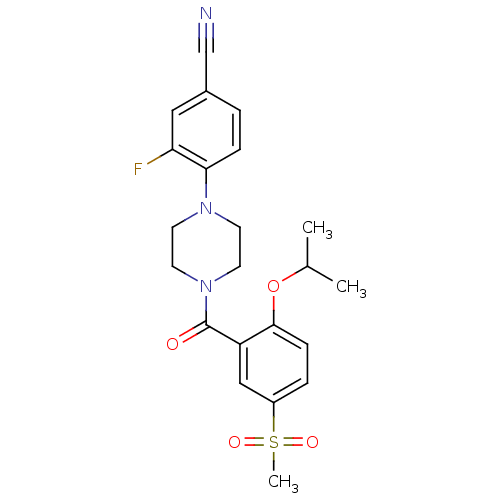
(3-Fluoro-4-[4-(2-isopropoxy-5-methanesulfonyl-benz...)Show SMILES CC(C)Oc1ccc(cc1C(=O)N1CCN(CC1)c1ccc(cc1F)C#N)S(C)(=O)=O Show InChI InChI=1S/C22H24FN3O4S/c1-15(2)30-21-7-5-17(31(3,28)29)13-18(21)22(27)26-10-8-25(9-11-26)20-6-4-16(14-24)12-19(20)23/h4-7,12-13,15H,8-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch clamp assay |
J Med Chem 53: 4603-14 (2010)
Article DOI: 10.1021/jm100210p
BindingDB Entry DOI: 10.7270/Q28916TT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188810
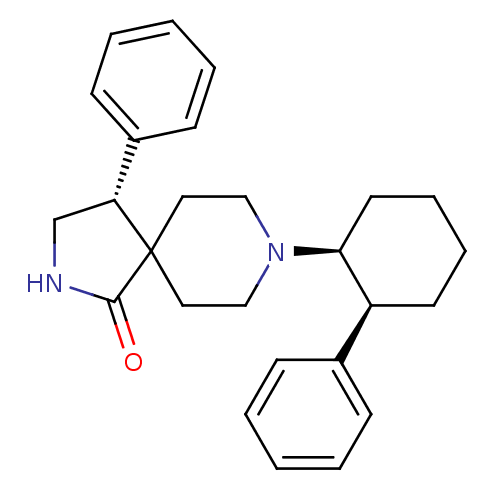
((R)-4-phenyl-8-((1S,2S)-2-phenyl-cyclohexyl)-2,8-d...)Show SMILES O=C1NC[C@H](c2ccccc2)C11CCN(CC1)[C@H]1CCCC[C@H]1c1ccccc1 Show InChI InChI=1S/C26H32N2O/c29-25-26(23(19-27-25)21-11-5-2-6-12-21)15-17-28(18-16-26)24-14-8-7-13-22(24)20-9-3-1-4-10-20/h1-6,9-12,22-24H,7-8,13-19H2,(H,27,29)/t22-,23+,24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4305-10 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.064
BindingDB Entry DOI: 10.7270/Q28C9VVV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188859
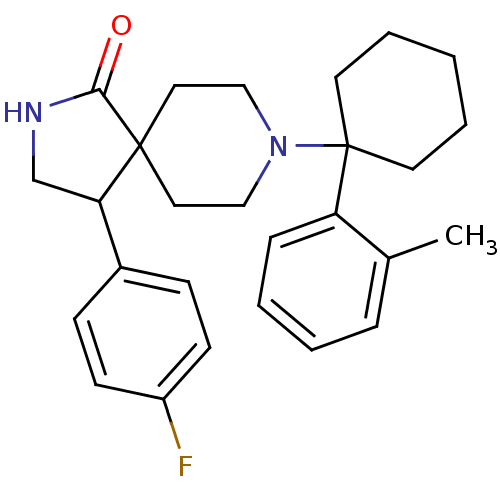
(CHEMBL378665 | rac-4-(4-fluoro-phenyl)-8-(1-o-toly...)Show SMILES Cc1ccccc1C1(CCCCC1)N1CCC2(CC1)C(CNC2=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H33FN2O/c1-20-7-3-4-8-23(20)27(13-5-2-6-14-27)30-17-15-26(16-18-30)24(19-29-25(26)31)21-9-11-22(28)12-10-21/h3-4,7-12,24H,2,5-6,13-19H2,1H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4321-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.063
BindingDB Entry DOI: 10.7270/Q2P26ZXG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50188803

((S)-4-phenyl-8-((1R,2R)-2-phenyl-cyclohexyl)-2,8-d...)Show SMILES O=C1NC[C@@H](c2ccccc2)C11CCN(CC1)[C@@H]1CCCC[C@@H]1c1ccccc1 Show InChI InChI=1S/C26H32N2O/c29-25-26(23(19-27-25)21-11-5-2-6-12-21)15-17-28(18-16-26)24-14-8-7-13-22(24)20-9-3-1-4-10-20/h1-6,9-12,22-24H,7-8,13-19H2,(H,27,29)/t22-,23+,24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human mu opioid receptor expressed in BHK cells |
Bioorg Med Chem Lett 16: 4311-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.058
BindingDB Entry DOI: 10.7270/Q2Z31Z8W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data