| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Leukotriene A-4 hydrolase |
|---|
| Ligand | BDBM50294167 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_571718 (CHEMBL1032963) |
|---|
| IC50 | 321000±n/a nM |
|---|
| Citation |  Davies, DR; Mamat, B; Magnusson, OT; Christensen, J; Haraldsson, MH; Mishra, R; Pease, B; Hansen, E; Singh, J; Zembower, D; Kim, H; Kiselyov, AS; Burgin, AB; Gurney, ME; Stewart, LJ Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. J Med Chem52:4694-715 (2009) [PubMed] Article Davies, DR; Mamat, B; Magnusson, OT; Christensen, J; Haraldsson, MH; Mishra, R; Pease, B; Hansen, E; Singh, J; Zembower, D; Kim, H; Kiselyov, AS; Burgin, AB; Gurney, ME; Stewart, LJ Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. J Med Chem52:4694-715 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Leukotriene A-4 hydrolase |
|---|
| Name: | Leukotriene A-4 hydrolase |
|---|
| Synonyms: | LKHA4_HUMAN | LTA-4 hydrolase | LTA4 | LTA4H | Leukotriene A(4) hydrolase | Leukotriene A-4 hydrolase (LTA4H) | Leukotriene A4 hydrolase |
|---|
| Type: | Hydrolase; metalloprotease |
|---|
| Mol. Mass.: | 69280.41 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human recombinant LTA4H. |
|---|
| Residue: | 611 |
|---|
| Sequence: | MPEIVDTCSLASPASVCRTKHLHLRCSVDFTRRTLTGTAALTVQSQEDNLRSLVLDTKDL
TIEKVVINGQEVKYALGERQSYKGSPMEISLPIALSKNQEIVIEISFETSPKSSALQWLT
PEQTSGKEHPYLFSQCQAIHCRAILPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGETP
DPEDPSRKIYKFIQKVPIPCYLIALVVGALESRQIGPRTLVWSEKEQVEKSAYEFSETES
MLKIAEDLGGPYVWGQYDLLVLPPSFPYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISH
SWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFNALGGWGELQNSVKTFGET
HPFTKLVVDLTDIDPDVAYSSVPYEKGFALLFYLEQLLGGPEIFLGFLKAYVEKFSYKSI
TTDDWKDFLYSYFKDKVDVLNQVDWNAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAK
EDDLNSFNATDLKDLSSHQLNEFLAQTLQRAPLPLGHIKRMQEVYNFNAINNSEIRFRWL
RLCIQSKWEDAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAVRTYQEHKASMHPVT
AMLVGKDLKVD
|
|
|
|---|
| BDBM50294167 |
|---|
| n/a |
|---|
| Name | BDBM50294167 |
|---|
| Synonyms: | 5-(2-(pyrrolidin-1-yl)ethoxy)-1H-indole | CHEMBL563692 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H18N2O |
|---|
| Mol. Mass. | 230.3055 |
|---|
| SMILES | C(CN1CCCC1)Oc1ccc2[nH]ccc2c1 |
|---|
| Structure | 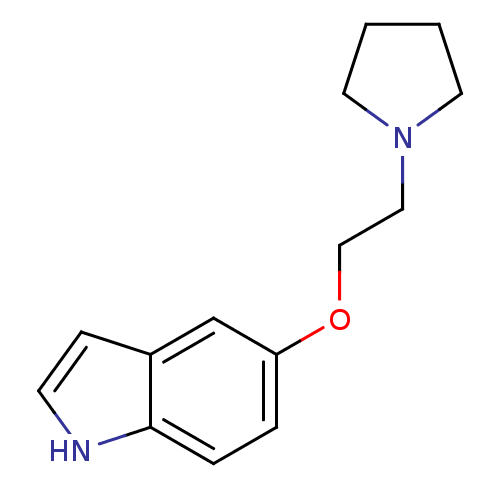
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Davies, DR; Mamat, B; Magnusson, OT; Christensen, J; Haraldsson, MH; Mishra, R; Pease, B; Hansen, E; Singh, J; Zembower, D; Kim, H; Kiselyov, AS; Burgin, AB; Gurney, ME; Stewart, LJ Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. J Med Chem52:4694-715 (2009) [PubMed] Article
Davies, DR; Mamat, B; Magnusson, OT; Christensen, J; Haraldsson, MH; Mishra, R; Pease, B; Hansen, E; Singh, J; Zembower, D; Kim, H; Kiselyov, AS; Burgin, AB; Gurney, ME; Stewart, LJ Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. J Med Chem52:4694-715 (2009) [PubMed] Article