| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine/threonine-protein phosphatase 2A activator |
|---|
| Ligand | BDBM50127375 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_161790 |
|---|
| IC50 | 4000±n/a nM |
|---|
| Citation |  Liu, W; Sheppeck, JE; Colby, DA; Huang, HB; Nairn, AC; Chamberlin, AR The selective inhibition of phosphatases by natural toxins: the anhydride domain of tautomycin is not a primary factor in controlling PP1/PP2A selectivity. Bioorg Med Chem Lett13:1597-600 (2003) [PubMed] Liu, W; Sheppeck, JE; Colby, DA; Huang, HB; Nairn, AC; Chamberlin, AR The selective inhibition of phosphatases by natural toxins: the anhydride domain of tautomycin is not a primary factor in controlling PP1/PP2A selectivity. Bioorg Med Chem Lett13:1597-600 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Serine/threonine-protein phosphatase 2A activator |
|---|
| Name: | Serine/threonine-protein phosphatase 2A activator |
|---|
| Synonyms: | 5.2.1.8 | PPP2R4 | PTPA | PTPA_HUMAN | Phosphotyrosyl phosphatase activator | Protein phosphatase 2A regulatory subunit B | Protein phosphatase 2A regulatory subunit B' | Serine/threonine-protein phosphatase 2A activator | Serine/threonine-protein phosphatase 2A regulatory subunit 4 |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 40662.53 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q15257 |
|---|
| Residue: | 358 |
|---|
| Sequence: | MAEGERQPPPDSSEEAPPATQNFIIPKKEIHTVPDMGKWKRSQAYADYIGFILTLNEGVK
GKKLTFEYRVSEMWNEVHEEKEQAAKQSVSCDECIPLPRAGHCAPSEAIEKLVALLNTLD
RWIDETPPVDQPSRFGNKAYRTWYAKLDEEAENLVATVVPTHLAAAVPEVAVYLKESVGN
STRIDYGTGHEAAFAAFLCCLCKIGVLRVDDQIAIVFKVFNRYLEVMRKLQKTYRMEPAG
SQGVWGLDDFQFLPFIWGSSQLIDHPYLEPRHFVDEKAVNENHKDYMFLECILFITEMKT
GPFAEHSNQLWNISAVPSWSKVNQGLIRMYKAECLEKFPVIQHFKFGSLLPIHPVTSG
|
|
|
|---|
| BDBM50127375 |
|---|
| n/a |
|---|
| Name | BDBM50127375 |
|---|
| Synonyms: | (E)-(R)-4-Hydroxy-2-methyl-hex-2-enedioic acid 6-{(1R,2S,3R,6S,7S,10R)-10-[(2S,3S,6R,8S,9R)-3,9-dimethyl-8-((S)-3-methyl-4-oxo-pentyl)-1,7-dioxa-spiro[5.5]undec-2-yl]-3,7-dihydroxy-1-isopropyl-2-methoxy-6-methyl-5-oxo-undecyl} ester | CHEMBL295239 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C40H68O12 |
|---|
| Mol. Mass. | 740.9607 |
|---|
| SMILES | CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)\C=C(/C)C(O)=O)C(C)C |
|---|
| Structure | 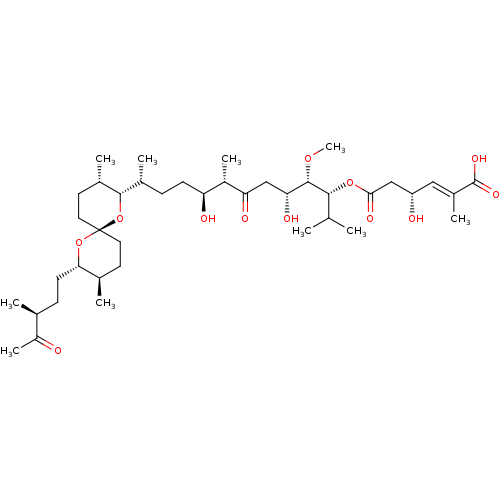
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, W; Sheppeck, JE; Colby, DA; Huang, HB; Nairn, AC; Chamberlin, AR The selective inhibition of phosphatases by natural toxins: the anhydride domain of tautomycin is not a primary factor in controlling PP1/PP2A selectivity. Bioorg Med Chem Lett13:1597-600 (2003) [PubMed]
Liu, W; Sheppeck, JE; Colby, DA; Huang, HB; Nairn, AC; Chamberlin, AR The selective inhibition of phosphatases by natural toxins: the anhydride domain of tautomycin is not a primary factor in controlling PP1/PP2A selectivity. Bioorg Med Chem Lett13:1597-600 (2003) [PubMed]