 Found 109 hits with Last Name = 'chamberlin' and Initial = 'ar'
Found 109 hits with Last Name = 'chamberlin' and Initial = 'ar' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50135699
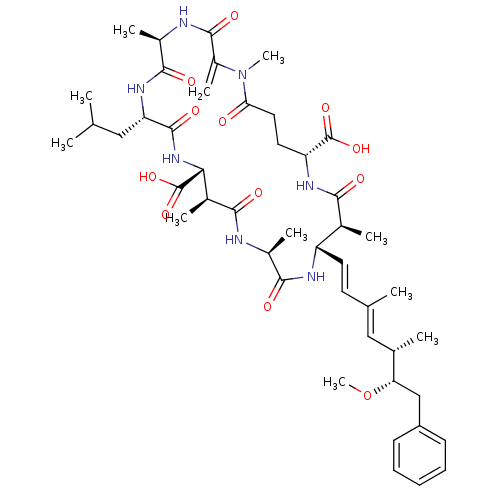
((10S,13S,18S,19S,22R)-8-Isobutyl-18-((1E,3E)-(5S,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C46H67N7O12/c1-24(2)21-35-44(60)52-38(46(63)64)28(6)40(56)47-29(7)41(57)49-33(18-17-25(3)22-26(4)36(65-11)23-32-15-13-12-14-16-32)27(5)39(55)50-34(45(61)62)19-20-37(54)53(10)31(9)43(59)48-30(8)42(58)51-35/h12-18,22,24,26-30,33-36,38H,9,19-21,23H2,1-8,10-11H3,(H,47,56)(H,48,59)(H,49,57)(H,50,55)(H,51,58)(H,52,60)(H,61,62)(H,63,64)/b18-17+,25-22+/t26-,27-,28-,29-,30+,33-,34+,35-,36-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 1 (PP1) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135699

((10S,13S,18S,19S,22R)-8-Isobutyl-18-((1E,3E)-(5S,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C46H67N7O12/c1-24(2)21-35-44(60)52-38(46(63)64)28(6)40(56)47-29(7)41(57)49-33(18-17-25(3)22-26(4)36(65-11)23-32-15-13-12-14-16-32)27(5)39(55)50-34(45(61)62)19-20-37(54)53(10)31(9)43(59)48-30(8)42(58)51-35/h12-18,22,24,26-30,33-36,38H,9,19-21,23H2,1-8,10-11H3,(H,47,56)(H,48,59)(H,49,57)(H,50,55)(H,51,58)(H,52,60)(H,61,62)(H,63,64)/b18-17+,25-22+/t26-,27-,28-,29-,30+,33-,34+,35-,36-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 1 (PP1) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50135699

((10S,13S,18S,19S,22R)-8-Isobutyl-18-((1E,3E)-(5S,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C46H67N7O12/c1-24(2)21-35-44(60)52-38(46(63)64)28(6)40(56)47-29(7)41(57)49-33(18-17-25(3)22-26(4)36(65-11)23-32-15-13-12-14-16-32)27(5)39(55)50-34(45(61)62)19-20-37(54)53(10)31(9)43(59)48-30(8)42(58)51-35/h12-18,22,24,26-30,33-36,38H,9,19-21,23H2,1-8,10-11H3,(H,47,56)(H,48,59)(H,49,57)(H,50,55)(H,51,58)(H,52,60)(H,61,62)(H,63,64)/b18-17+,25-22+/t26-,27-,28-,29-,30+,33-,34+,35-,36-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 2A (PP2A) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135699

((10S,13S,18S,19S,22R)-8-Isobutyl-18-((1E,3E)-(5S,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C46H67N7O12/c1-24(2)21-35-44(60)52-38(46(63)64)28(6)40(56)47-29(7)41(57)49-33(18-17-25(3)22-26(4)36(65-11)23-32-15-13-12-14-16-32)27(5)39(55)50-34(45(61)62)19-20-37(54)53(10)31(9)43(59)48-30(8)42(58)51-35/h12-18,22,24,26-30,33-36,38H,9,19-21,23H2,1-8,10-11H3,(H,47,56)(H,48,59)(H,49,57)(H,50,55)(H,51,58)(H,52,60)(H,61,62)(H,63,64)/b18-17+,25-22+/t26-,27-,28-,29-,30+,33-,34+,35-,36-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 2A using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1A
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135691
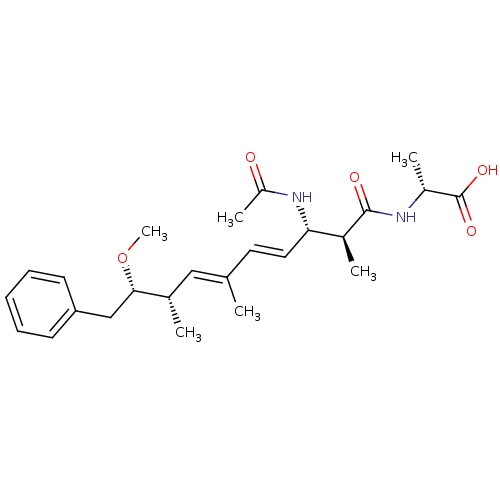
((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 2A (PP2A) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083523

((1-Benzyl-1H-quinolin-4-ylidene)-pentyl-amine | CH...)Show InChI InChI=1S/C21H24N2/c1-2-3-9-15-22-20-14-16-23(17-18-10-5-4-6-11-18)21-13-8-7-12-19(20)21/h4-8,10-14,16H,2-3,9,15,17H2,1H3/b22-20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176903
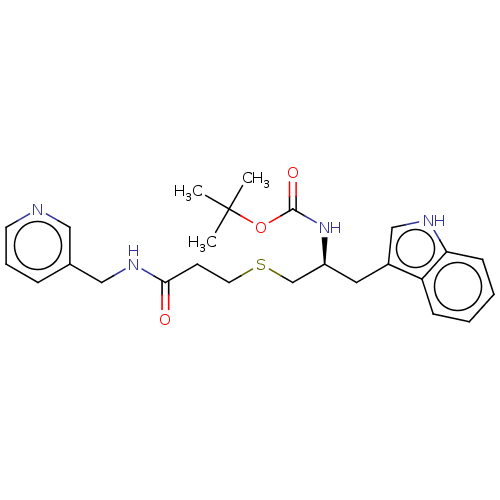
(CHEMBL3814877)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H32N4O3S/c1-25(2,3)32-24(31)29-20(13-19-16-27-22-9-5-4-8-21(19)22)17-33-12-10-23(30)28-15-18-7-6-11-26-14-18/h4-9,11,14,16,20,27H,10,12-13,15,17H2,1-3H3,(H,28,30)(H,29,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50090505
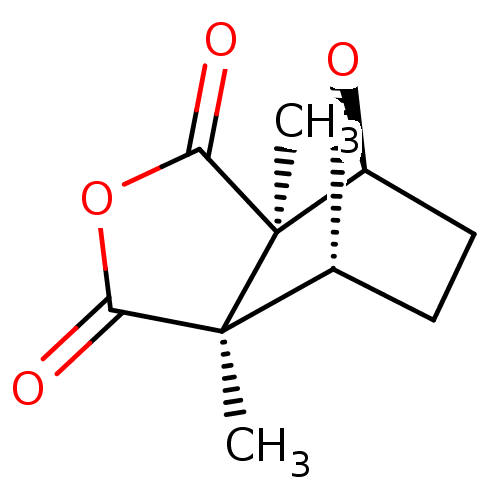
((1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxa-tricyclo[5.2...)Show SMILES C[C@]12[C@@H]3CC[C@@H](O3)[C@@]1(C)C(=O)OC2=O Show InChI InChI=1S/C10H12O4/c1-9-5-3-4-6(13-5)10(9,2)8(12)14-7(9)11/h5-6H,3-4H2,1-2H3/t5-,6+,9+,10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A activator
(Homo sapiens (Human)) | BDBM50127377

(10-[3,9-dimethyl-8-(3-methyl-4-oxopentyl)-(9R)-1,7...)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@H](O)C1=C(C)C(=O)OC1=O)C(C)C |c:45| Show InChI InChI=1S/C41H66O13/c1-21(2)36(51-34(47)20-31(45)35-27(8)39(48)52-40(35)49)38(50-10)32(46)19-30(44)26(7)29(43)13-11-24(5)37-25(6)16-18-41(54-37)17-15-23(4)33(53-41)14-12-22(3)28(9)42/h21-26,29,31-33,36-38,43,45-46H,11-20H2,1-10H3/t22-,23+,24+,25-,26-,29-,31-,32+,33-,36+,37-,38-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay |
Bioorg Med Chem Lett 13: 1597-600 (2003)
BindingDB Entry DOI: 10.7270/Q2DB82CK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135697
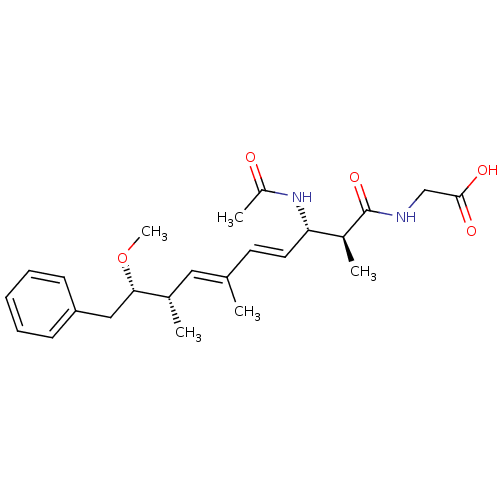
(((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-methoxy-2,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)NCC(O)=O Show InChI InChI=1S/C24H34N2O5/c1-16(13-17(2)22(31-5)14-20-9-7-6-8-10-20)11-12-21(26-19(4)27)18(3)24(30)25-15-23(28)29/h6-13,17-18,21-22H,14-15H2,1-5H3,(H,25,30)(H,26,27)(H,28,29)/b12-11+,16-13+/t17-,18-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 2A (PP2A) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176904
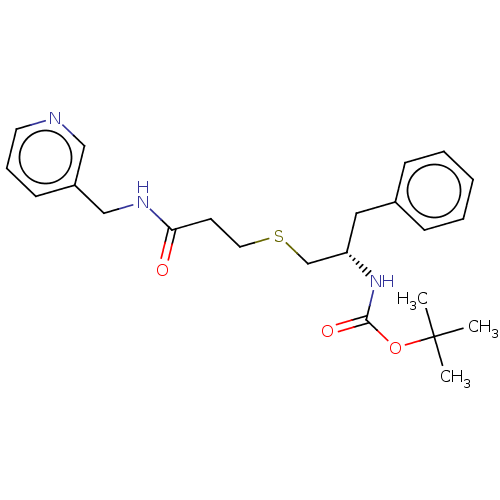
(CHEMBL3814479)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C23H31N3O3S/c1-23(2,3)29-22(28)26-20(14-18-8-5-4-6-9-18)17-30-13-11-21(27)25-16-19-10-7-12-24-15-19/h4-10,12,15,20H,11,13-14,16-17H2,1-3H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50366474

(NORCANTHARIDIN)Show SMILES O=C1OC(=O)[C@@H]2[C@H]3CC[C@@H](O3)[C@H]12 |r,TLB:1:11:8.7:10,THB:3:5:8.7:10| Show InChI InChI=1S/C8H8O4/c9-7-5-3-1-2-4(11-3)6(5)8(10)12-7/h3-6H,1-2H2/t3-,4-,5-,6+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50135691

((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19+,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 1 (PP1) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083514
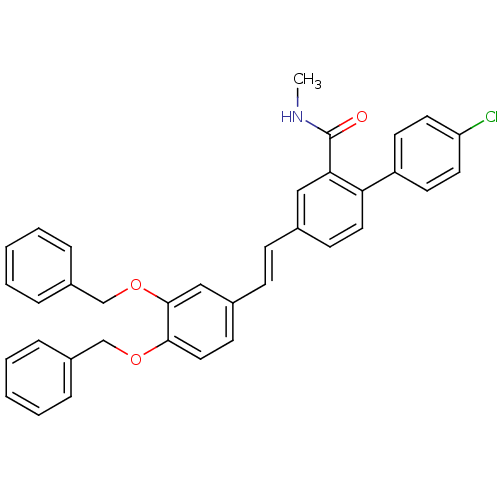
(4-[(E)-2-(3,4-Bis-benzyloxy-phenyl)-vinyl]-4'-chlo...)Show SMILES CNC(=O)c1cc(\C=C\c2ccc(OCc3ccccc3)c(OCc3ccccc3)c2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C36H30ClNO3/c1-38-36(39)33-22-26(14-20-32(33)30-16-18-31(37)19-17-30)12-13-27-15-21-34(40-24-28-8-4-2-5-9-28)35(23-27)41-25-29-10-6-3-7-11-29/h2-23H,24-25H2,1H3,(H,38,39)/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083518

(4'-Chloro-4-((E)-4-phenyl-but-1-enyl)-biphenyl-2-c...)Show SMILES CCNC(=O)c1cc(\C=C\CCc2ccccc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClNO/c1-2-27-25(28)24-18-20(11-7-6-10-19-8-4-3-5-9-19)12-17-23(24)21-13-15-22(26)16-14-21/h3-5,7-9,11-18H,2,6,10H2,1H3,(H,27,28)/b11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083522
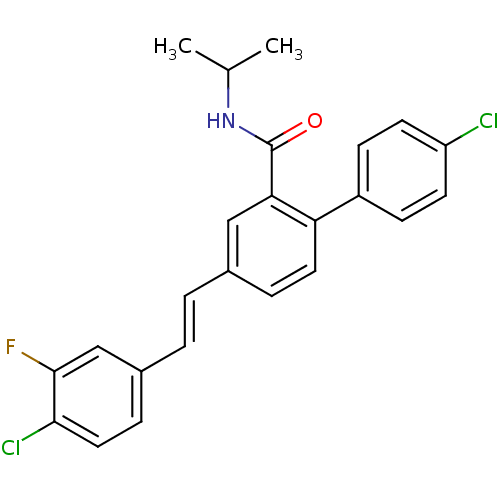
(4'-Chloro-4-[(E)-2-(4-chloro-3-fluoro-phenyl)-viny...)Show SMILES CC(C)NC(=O)c1cc(\C=C\c2ccc(Cl)c(F)c2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2FNO/c1-15(2)28-24(29)21-13-16(3-4-17-6-12-22(26)23(27)14-17)5-11-20(21)18-7-9-19(25)10-8-18/h3-15H,1-2H3,(H,28,29)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083515
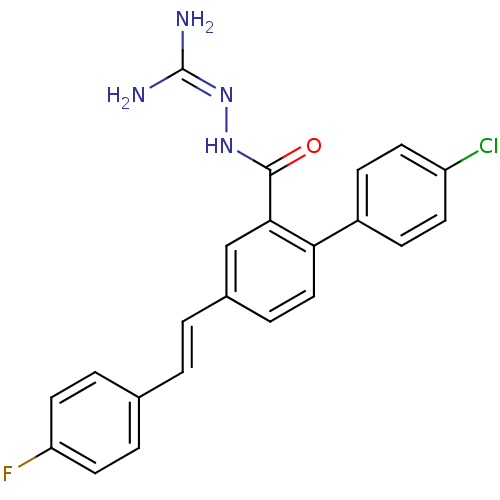
(2-({4'-chloro-4-[(E)-2-(4-fluorophenyl)vinyl]-1,1'...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]-[#6](=O)-c1cc(\[#6]=[#6]\c2ccc(F)cc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H18ClFN4O/c23-17-8-6-16(7-9-17)19-12-5-15(2-1-14-3-10-18(24)11-4-14)13-20(19)21(29)27-28-22(25)26/h1-13H,(H,27,29)(H4,25,26,28)/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
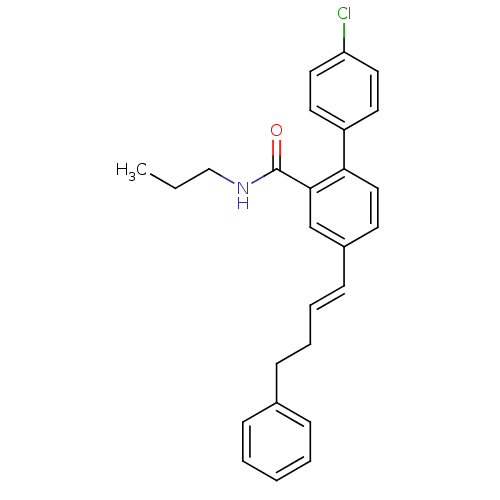
(Homo sapiens (Human)) | BDBM50083513
(4'-Chloro-4-((E)-4-phenyl-but-1-enyl)-biphenyl-2-c...)Show SMILES CCCNC(=O)c1cc(\C=C\CCc2ccccc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H26ClNO/c1-2-18-28-26(29)25-19-21(11-7-6-10-20-8-4-3-5-9-20)12-17-24(25)22-13-15-23(27)16-14-22/h3-5,7-9,11-17,19H,2,6,10,18H2,1H3,(H,28,29)/b11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A activator
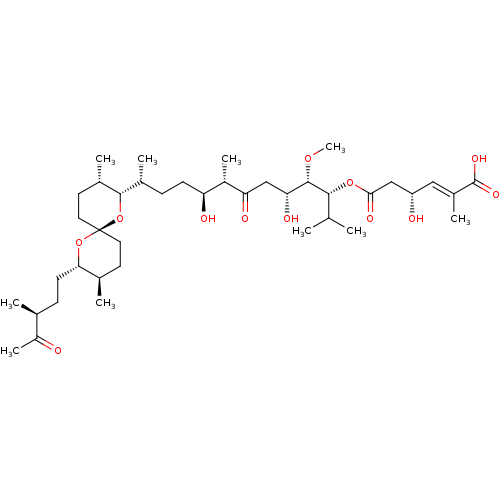
(Homo sapiens (Human)) | BDBM50127375
((E)-(R)-4-Hydroxy-2-methyl-hex-2-enedioic acid 6-{...)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)\C=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C40H68O12/c1-22(2)36(50-35(46)20-30(42)19-27(7)39(47)48)38(49-10)33(45)21-32(44)28(8)31(43)13-11-25(5)37-26(6)16-18-40(52-37)17-15-24(4)34(51-40)14-12-23(3)29(9)41/h19,22-26,28,30-31,33-34,36-38,42-43,45H,11-18,20-21H2,1-10H3,(H,47,48)/b27-19+/t23-,24+,25+,26-,28-,30-,31-,33+,34-,36+,37-,38-,40+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay |
Bioorg Med Chem Lett 13: 1597-600 (2003)
BindingDB Entry DOI: 10.7270/Q2DB82CK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083511

(4'-Chloro-4-((E)-3-phenyl-propenyl)-biphenyl-2-car...)Show SMILES CC(C)NC(=O)c1cc(\C=C\Cc2ccccc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClNO/c1-18(2)27-25(28)24-17-20(10-6-9-19-7-4-3-5-8-19)11-16-23(24)21-12-14-22(26)15-13-21/h3-8,10-18H,9H2,1-2H3,(H,27,28)/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50135697

(((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-methoxy-2,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)NCC(O)=O Show InChI InChI=1S/C24H34N2O5/c1-16(13-17(2)22(31-5)14-20-9-7-6-8-10-20)11-12-21(26-19(4)27)18(3)24(30)25-15-23(28)29/h6-13,17-18,21-22H,14-15H2,1-5H3,(H,25,30)(H,26,27)(H,28,29)/b12-11+,16-13+/t17-,18-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 1 (PP1) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
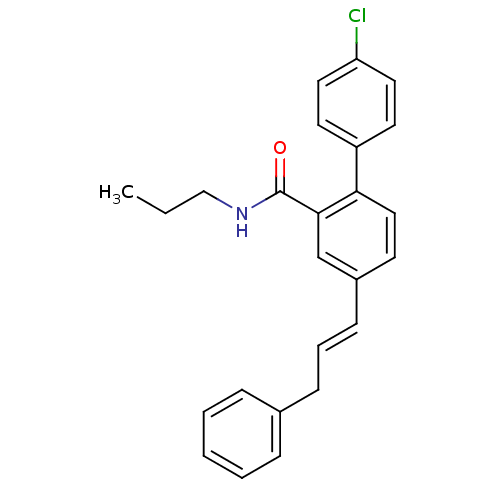
(Homo sapiens (Human)) | BDBM50083516
(4'-Chloro-4-((E)-3-phenyl-propenyl)-biphenyl-2-car...)Show SMILES CCCNC(=O)c1cc(\C=C\Cc2ccccc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClNO/c1-2-17-27-25(28)24-18-20(10-6-9-19-7-4-3-5-8-19)11-16-23(24)21-12-14-22(26)15-13-21/h3-8,10-16,18H,2,9,17H2,1H3,(H,27,28)/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083519
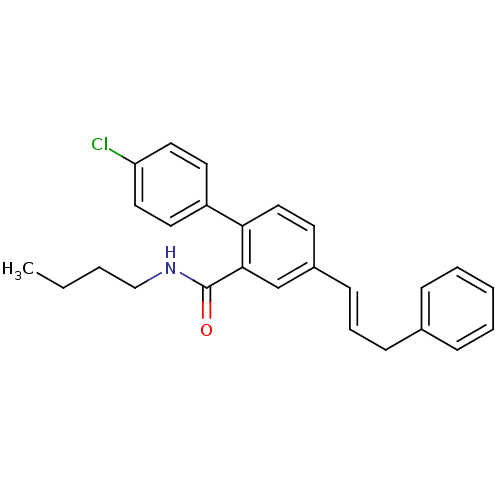
(4'-Chloro-4-((E)-3-phenyl-propenyl)-biphenyl-2-car...)Show SMILES CCCCNC(=O)c1cc(\C=C\Cc2ccccc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H26ClNO/c1-2-3-18-28-26(29)25-19-21(11-7-10-20-8-5-4-6-9-20)12-17-24(25)22-13-15-23(27)16-14-22/h4-9,11-17,19H,2-3,10,18H2,1H3,(H,28,29)/b11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135691

((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135700
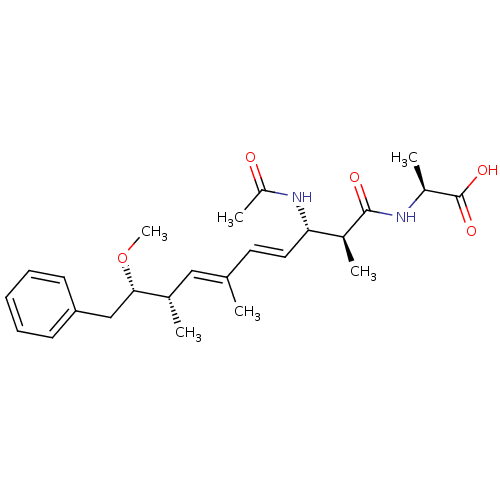
((S)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 2A (PP2A) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083517
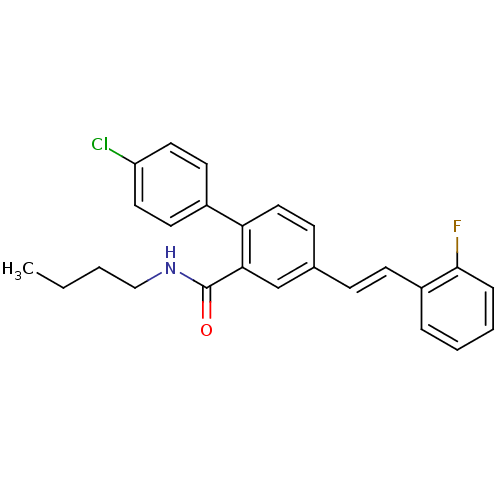
(4'-Chloro-4-[(E)-2-(2-fluoro-phenyl)-vinyl]-biphen...)Show SMILES CCCCNC(=O)c1cc(\C=C\c2ccccc2F)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H23ClFNO/c1-2-3-16-28-25(29)23-17-18(8-10-20-6-4-5-7-24(20)27)9-15-22(23)19-11-13-21(26)14-12-19/h4-15,17H,2-3,16H2,1H3,(H,28,29)/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A activator
(Homo sapiens (Human)) | BDBM50366883
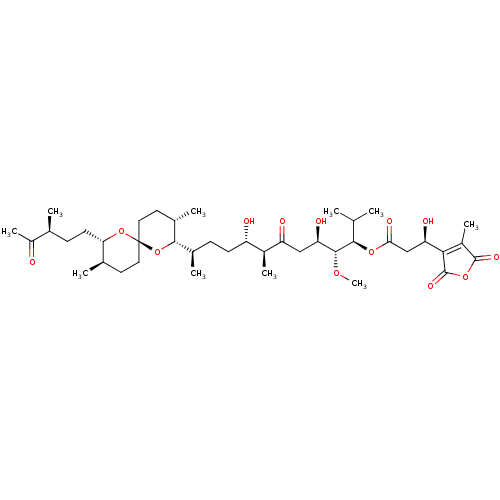
(TAUTOMYCIN)Show SMILES CO[C@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C1=C(C)C(=O)OC1=O)C(C)C |r,c:45| Show InChI InChI=1S/C41H66O13/c1-21(2)36(51-34(47)20-31(45)35-27(8)39(48)52-40(35)49)38(50-10)32(46)19-30(44)26(7)29(43)13-11-24(5)37-25(6)16-18-41(54-37)17-15-23(4)33(53-41)14-12-22(3)28(9)42/h21-26,29,31-33,36-38,43,45-46H,11-20H2,1-10H3/t22-,23+,24+,25-,26-,29-,31+,32+,33-,36+,37-,38+,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay |
Bioorg Med Chem Lett 13: 1597-600 (2003)
BindingDB Entry DOI: 10.7270/Q2DB82CK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083512
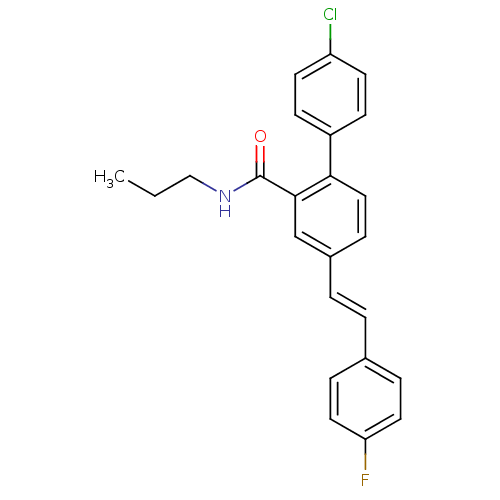
(4'-Chloro-4-[(E)-2-(4-fluoro-phenyl)-vinyl]-biphen...)Show SMILES CCCNC(=O)c1cc(\C=C\c2ccc(F)cc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClFNO/c1-2-15-27-24(28)23-16-18(4-3-17-5-12-21(26)13-6-17)7-14-22(23)19-8-10-20(25)11-9-19/h3-14,16H,2,15H2,1H3,(H,27,28)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
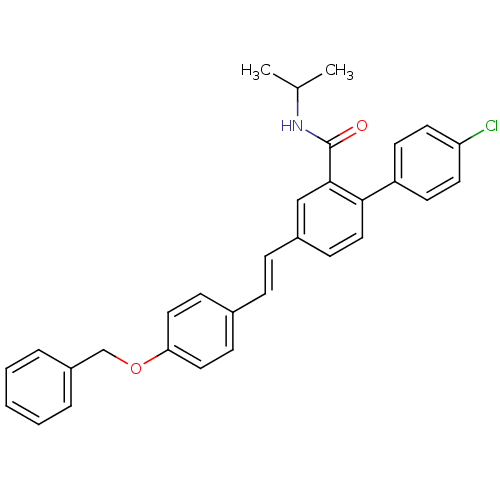
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083520
(4-[(E)-2-(4-Benzyloxy-phenyl)-vinyl]-4'-chloro-bip...)Show SMILES CC(C)NC(=O)c1cc(\C=C\c2ccc(OCc3ccccc3)cc2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C31H28ClNO2/c1-22(2)33-31(34)30-20-24(12-19-29(30)26-13-15-27(32)16-14-26)9-8-23-10-17-28(18-11-23)35-21-25-6-4-3-5-7-25/h3-20,22H,21H2,1-2H3,(H,33,34)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135696
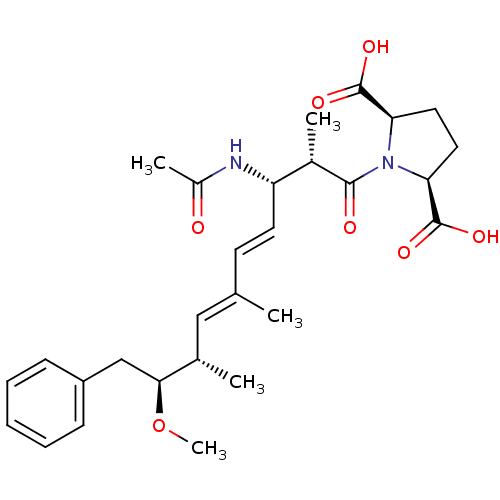
((2R,5S)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1[C@H](CC[C@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C28H38N2O7/c1-17(15-18(2)25(37-5)16-21-9-7-6-8-10-21)11-12-22(29-20(4)31)19(3)26(32)30-23(27(33)34)13-14-24(30)28(35)36/h6-12,15,18-19,22-25H,13-14,16H2,1-5H3,(H,29,31)(H,33,34)(H,35,36)/b12-11+,17-15+/t18-,19-,22-,23-,24+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50083521
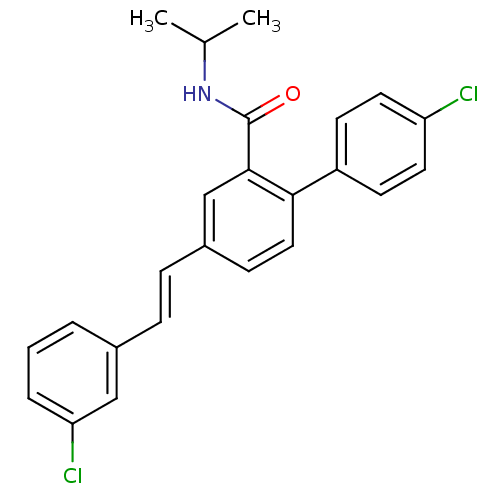
(4'-Chloro-4-[(E)-2-(3-chloro-phenyl)-vinyl]-biphen...)Show SMILES CC(C)NC(=O)c1cc(\C=C\c2cccc(Cl)c2)ccc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21Cl2NO/c1-16(2)27-24(28)23-15-18(7-6-17-4-3-5-21(26)14-17)8-13-22(23)19-9-11-20(25)12-10-19/h3-16H,1-2H3,(H,27,28)/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ChTX from human T cell voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 9: 3267-72 (2000)
BindingDB Entry DOI: 10.7270/Q2765DJW |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50143522
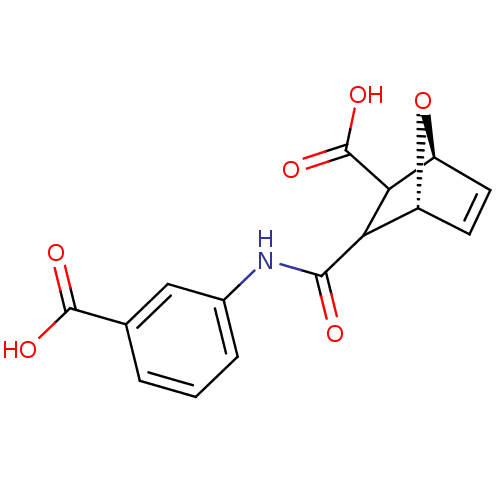
((1S,4R)-3-(3-Carboxy-phenylcarbamoyl)-7-oxa-bicycl...)Show SMILES OC(=O)C1[C@H]2O[C@H](C=C2)C1C(=O)Nc1cccc(c1)C(O)=O |c:7| Show InChI InChI=1S/C15H13NO6/c17-13(16-8-3-1-2-7(6-8)14(18)19)11-9-4-5-10(22-9)12(11)15(20)21/h1-6,9-12H,(H,16,17)(H,18,19)(H,20,21)/t9-,10+,11?,12?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50143517
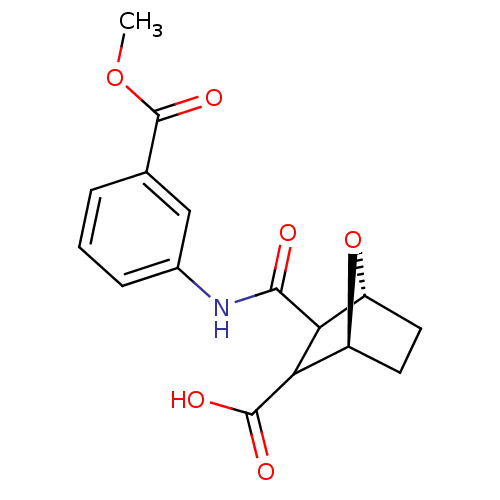
((1S,4R)-3-(3-Methoxycarbonyl-phenylcarbamoyl)-7-ox...)Show SMILES COC(=O)c1cccc(NC(=O)C2[C@H]3CC[C@H](O3)C2C(O)=O)c1 Show InChI InChI=1S/C16H17NO6/c1-22-16(21)8-3-2-4-9(7-8)17-14(18)12-10-5-6-11(23-10)13(12)15(19)20/h2-4,7,10-13H,5-6H2,1H3,(H,17,18)(H,19,20)/t10-,11+,12?,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50135676

((S)-2-[(R)-2-(2-{[(R)-4-((4E,6E)-(2S,3S,8S,9S)-3-A...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](CCC(=O)N(C)C(=C)C(=O)N[C@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O)C(O)=O Show InChI InChI=1S/C40H59N5O10/c1-23(2)20-33(40(53)54)44-37(49)27(6)41-38(50)28(7)45(9)35(47)19-18-32(39(51)52)43-36(48)26(5)31(42-29(8)46)17-16-24(3)21-25(4)34(55-10)22-30-14-12-11-13-15-30/h11-17,21,23,25-27,31-34H,7,18-20,22H2,1-6,8-10H3,(H,41,50)(H,42,46)(H,43,48)(H,44,49)(H,51,52)(H,53,54)/b17-16+,24-21+/t25-,26-,27+,31-,32+,33-,34-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 2A using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50135679
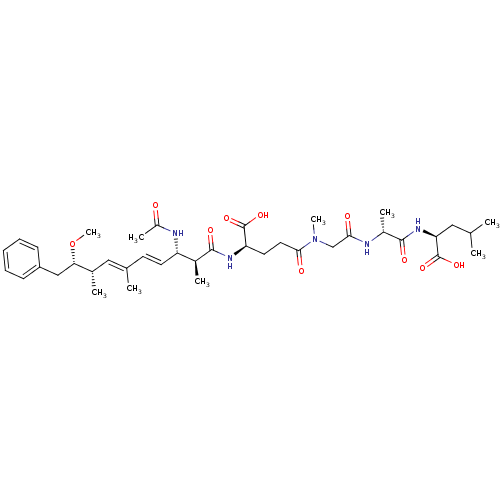
((S)-2-[(R)-2-(2-{[(R)-4-((4E,6E)-(2S,3S,8S,9S)-3-A...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](CCC(=O)N(C)CC(=O)N[C@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O)C(O)=O Show InChI InChI=1S/C39H59N5O10/c1-23(2)19-32(39(52)53)43-37(49)27(6)40-34(46)22-44(8)35(47)18-17-31(38(50)51)42-36(48)26(5)30(41-28(7)45)16-15-24(3)20-25(4)33(54-9)21-29-13-11-10-12-14-29/h10-16,20,23,25-27,30-33H,17-19,21-22H2,1-9H3,(H,40,46)(H,41,45)(H,42,48)(H,43,49)(H,50,51)(H,52,53)/b16-15+,24-20+/t25-,26-,27+,30-,31+,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 2A using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50143509
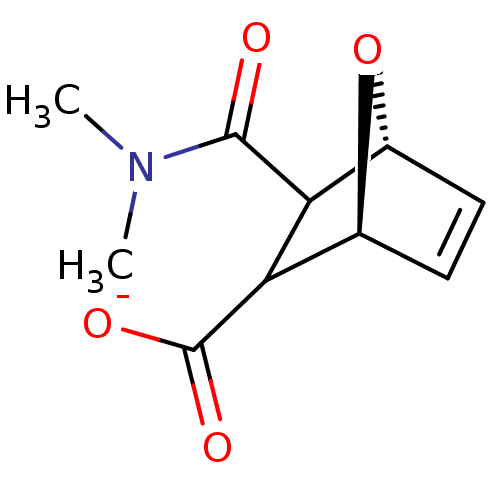
((1S,4R)-3-Dimethylcarbamoyl-7-oxa-bicyclo[2.2.1]he...)Show SMILES CN(C)C(=O)C1[C@@H]2O[C@@H](C=C2)C1C([O-])=O |c:9| Show InChI InChI=1S/C10H13NO4/c1-11(2)9(12)7-5-3-4-6(15-5)8(7)10(13)14/h3-8H,1-2H3,(H,13,14)/p-1/t5-,6+,7?,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50135677
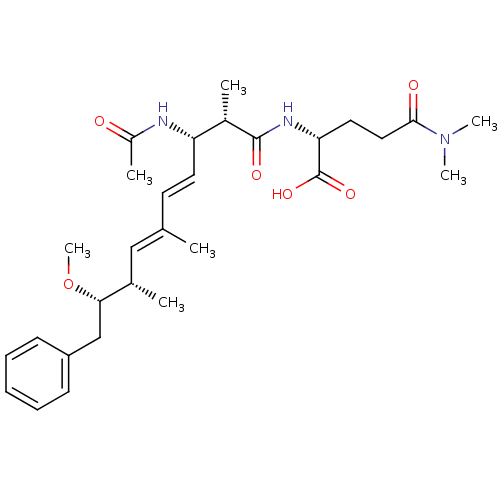
((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](CCC(=O)N(C)C)C(O)=O Show InChI InChI=1S/C29H43N3O6/c1-19(17-20(2)26(38-7)18-23-11-9-8-10-12-23)13-14-24(30-22(4)33)21(3)28(35)31-25(29(36)37)15-16-27(34)32(5)6/h8-14,17,20-21,24-26H,15-16,18H2,1-7H3,(H,30,33)(H,31,35)(H,36,37)/b14-13+,19-17+/t20-,21-,24-,25+,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 2A using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
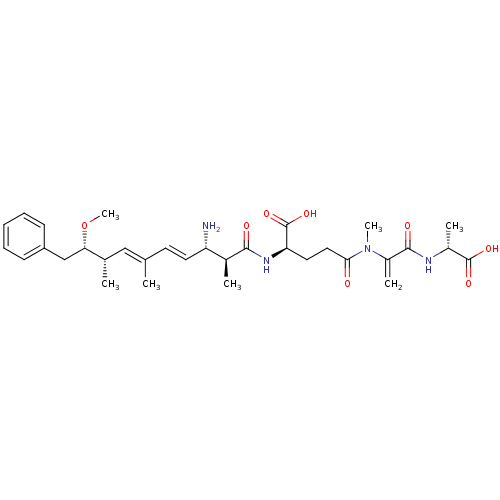
(Gallus gallus) | BDBM50135681
((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Amino-9-methoxy-2,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](N)[C@H](C)C(=O)N[C@H](CCC(=O)N(C)C(=C)C(=O)N[C@H](C)C(O)=O)C(O)=O Show InChI InChI=1S/C32H46N4O8/c1-19(17-20(2)27(44-7)18-24-11-9-8-10-12-24)13-14-25(33)21(3)29(38)35-26(32(42)43)15-16-28(37)36(6)23(5)30(39)34-22(4)31(40)41/h8-14,17,20-22,25-27H,5,15-16,18,33H2,1-4,6-7H3,(H,34,39)(H,35,38)(H,40,41)(H,42,43)/b14-13+,19-17+/t20-,21-,22+,25-,26+,27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 2A using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
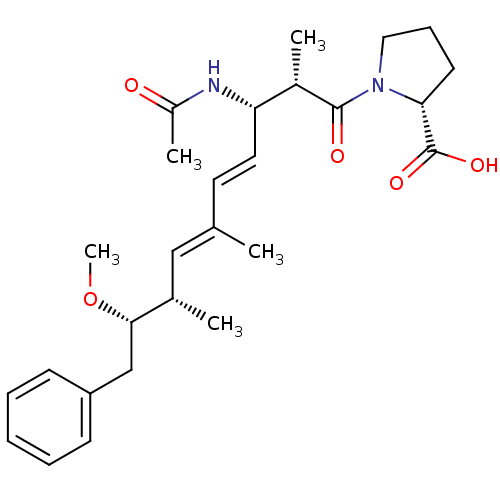
(Bos taurus) | BDBM50135687
((R)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1CCC[C@@H]1C(O)=O Show InChI InChI=1S/C27H38N2O5/c1-18(16-19(2)25(34-5)17-22-10-7-6-8-11-22)13-14-23(28-21(4)30)20(3)26(31)29-15-9-12-24(29)27(32)33/h6-8,10-11,13-14,16,19-20,23-25H,9,12,15,17H2,1-5H3,(H,28,30)(H,32,33)/b14-13+,18-16+/t19-,20-,23-,24+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50135691

((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19+,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 1 (PP1) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
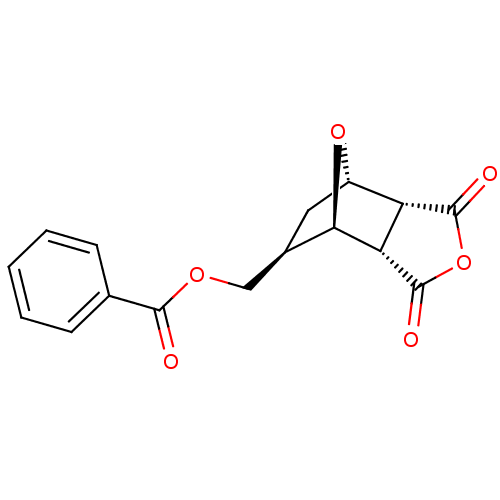
(Homo sapiens (Human)) | BDBM50143515
(Benzoic acid (1S,2R,6S,7R,8R)-3,5-dioxo-4,10-dioxa...)Show SMILES O=C(OC[C@H]1C[C@@H]2O[C@H]1[C@@H]1[C@H]2C(=O)OC1=O)c1ccccc1 Show InChI InChI=1S/C16H14O6/c17-14(8-4-2-1-3-5-8)20-7-9-6-10-11-12(13(9)21-10)16(19)22-15(11)18/h1-5,9-13H,6-7H2/t9-,10+,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50135675
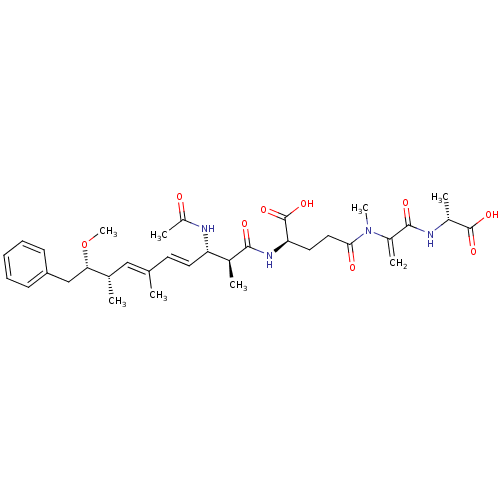
((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](CCC(=O)N(C)C(=C)C(=O)N[C@H](C)C(O)=O)C(O)=O Show InChI InChI=1S/C34H48N4O9/c1-20(18-21(2)29(47-8)19-26-12-10-9-11-13-26)14-15-27(36-25(6)39)22(3)31(41)37-28(34(45)46)16-17-30(40)38(7)24(5)32(42)35-23(4)33(43)44/h9-15,18,21-23,27-29H,5,16-17,19H2,1-4,6-8H3,(H,35,42)(H,36,39)(H,37,41)(H,43,44)(H,45,46)/b15-14+,20-18+/t21-,22-,23+,27-,28+,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 2A using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A activator
(Homo sapiens (Human)) | BDBM50127378

((S)-2-Hydroxy-2-methyl-succinic acid 4-{(1R,2S,3R,...)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@](C)(O)C(O)=O)C(C)C Show InChI InChI=1S/C38H66O12/c1-21(2)33(48-32(43)20-37(9,46)36(44)45)35(47-10)30(42)19-29(41)26(7)28(40)13-11-24(5)34-25(6)16-18-38(50-34)17-15-23(4)31(49-38)14-12-22(3)27(8)39/h21-26,28,30-31,33-35,40,42,46H,11-20H2,1-10H3,(H,44,45)/t22-,23+,24+,25-,26-,28-,30+,31-,33+,34-,35-,37-,38+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay |
Bioorg Med Chem Lett 13: 1597-600 (2003)
BindingDB Entry DOI: 10.7270/Q2DB82CK |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50143516
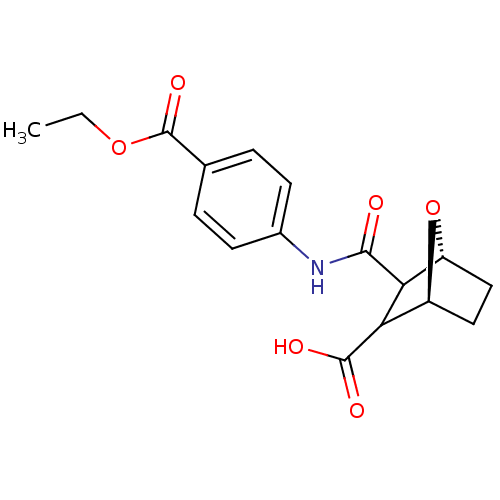
((1S,4R)-3-(4-Ethoxycarbonyl-phenylcarbamoyl)-7-oxa...)Show SMILES CCOC(=O)c1ccc(NC(=O)C2[C@H]3CC[C@H](O3)C2C(O)=O)cc1 Show InChI InChI=1S/C17H19NO6/c1-2-23-17(22)9-3-5-10(6-4-9)18-15(19)13-11-7-8-12(24-11)14(13)16(20)21/h3-6,11-14H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t11-,12+,13?,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176905
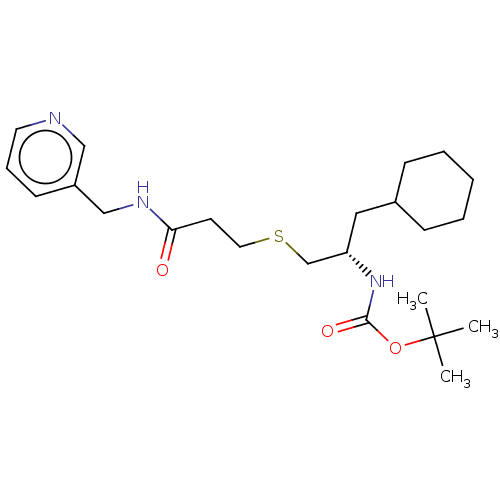
(CHEMBL3814345)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)CC1CCCCC1 |r| Show InChI InChI=1S/C23H37N3O3S/c1-23(2,3)29-22(28)26-20(14-18-8-5-4-6-9-18)17-30-13-11-21(27)25-16-19-10-7-12-24-15-19/h7,10,12,15,18,20H,4-6,8-9,11,13-14,16-17H2,1-3H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135685
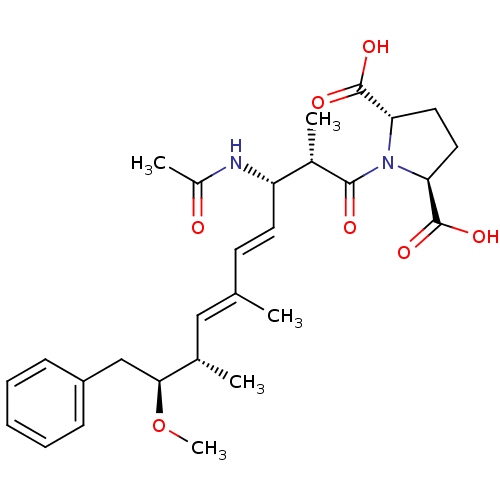
((2S,5S)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1[C@@H](CC[C@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C28H38N2O7/c1-17(15-18(2)25(37-5)16-21-9-7-6-8-10-21)11-12-22(29-20(4)31)19(3)26(32)30-23(27(33)34)13-14-24(30)28(35)36/h6-12,15,18-19,22-25H,13-14,16H2,1-5H3,(H,29,31)(H,33,34)(H,35,36)/b12-11+,17-15+/t18-,19-,22-,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1A
(Homo sapiens (Human)) | BDBM50135681

((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Amino-9-methoxy-2,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](N)[C@H](C)C(=O)N[C@H](CCC(=O)N(C)C(=C)C(=O)N[C@H](C)C(O)=O)C(O)=O Show InChI InChI=1S/C32H46N4O8/c1-19(17-20(2)27(44-7)18-24-11-9-8-10-12-24)13-14-25(33)21(3)29(38)35-26(32(42)43)15-16-28(37)36(6)23(5)30(39)34-22(4)31(40)41/h8-14,17,20-22,25-27H,5,15-16,18,33H2,1-4,6-7H3,(H,34,39)(H,35,38)(H,40,41)(H,42,43)/b14-13+,19-17+/t20-,21-,22+,25-,26+,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay |
Bioorg Med Chem Lett 13: 2903-6 (2003)
BindingDB Entry DOI: 10.7270/Q2X63MB4 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1B
(Homo sapiens (Human)) | BDBM50143512
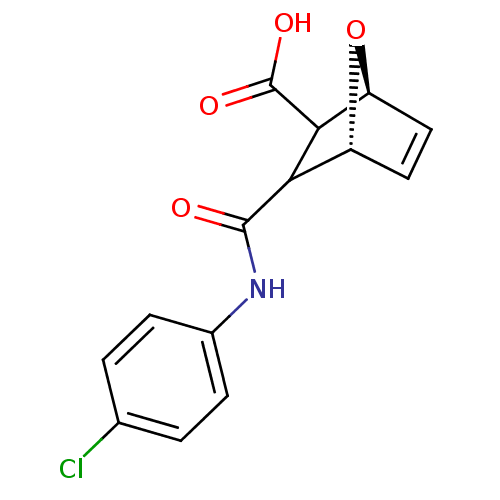
((1S,4R)-3-(4-Chloro-phenylcarbamoyl)-7-oxa-bicyclo...)Show SMILES OC(=O)C1[C@H]2O[C@H](C=C2)C1C(=O)Nc1ccc(Cl)cc1 |c:7| Show InChI InChI=1S/C14H12ClNO4/c15-7-1-3-8(4-2-7)16-13(17)11-9-5-6-10(20-9)12(11)14(18)19/h1-6,9-12H,(H,16,17)(H,18,19)/t9-,10+,11?,12?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 14: 1969-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.093
BindingDB Entry DOI: 10.7270/Q25H7GTJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data