| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50335379 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_701745 (CHEMBL1656748) |
|---|
| IC50 | 1600±n/a nM |
|---|
| Citation |  Johnson, DS; Stiff, C; Lazerwith, SE; Kesten, SR; Fay, LK; Morris, M; Beidler, D; Liimatta, MB; Smith, SE; Dudley, DT; Sadagopan, N; Bhattachar, SN; Kesten, SJ; Nomanbhoy, TK; Cravatt, BF; Ahn, K Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH Inhibitor. ACS Med Chem Lett2:91-96 (2011) [PubMed] Article Johnson, DS; Stiff, C; Lazerwith, SE; Kesten, SR; Fay, LK; Morris, M; Beidler, D; Liimatta, MB; Smith, SE; Dudley, DT; Sadagopan, N; Bhattachar, SN; Kesten, SJ; Nomanbhoy, TK; Cravatt, BF; Ahn, K Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH Inhibitor. ACS Med Chem Lett2:91-96 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50335379 |
|---|
| n/a |
|---|
| Name | BDBM50335379 |
|---|
| Synonyms: | CHEMBL1651532 | N-(6-Methylpyridin-3-yl)-4-(3-{[5-(trifluoromethyl)pyridin-2-yl]oxy}benzylidene)piperidine-1-carboxamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H23F3N4O2 |
|---|
| Mol. Mass. | 468.4709 |
|---|
| SMILES | [#6]-c1ccc(-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]\c2cccc(-[#8]-c3ccc(cn3)C(F)(F)F)c2)cn1 |
|---|
| Structure | 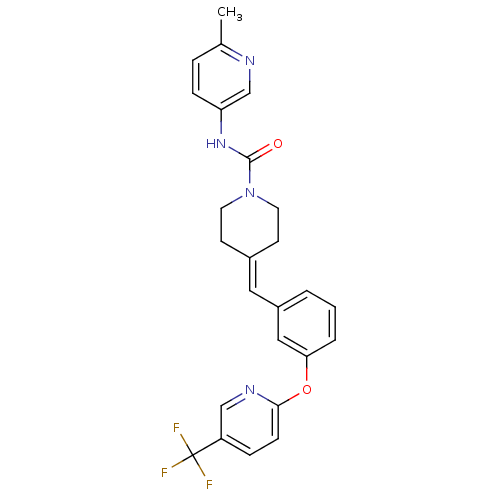
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Johnson, DS; Stiff, C; Lazerwith, SE; Kesten, SR; Fay, LK; Morris, M; Beidler, D; Liimatta, MB; Smith, SE; Dudley, DT; Sadagopan, N; Bhattachar, SN; Kesten, SJ; Nomanbhoy, TK; Cravatt, BF; Ahn, K Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH Inhibitor. ACS Med Chem Lett2:91-96 (2011) [PubMed] Article
Johnson, DS; Stiff, C; Lazerwith, SE; Kesten, SR; Fay, LK; Morris, M; Beidler, D; Liimatta, MB; Smith, SE; Dudley, DT; Sadagopan, N; Bhattachar, SN; Kesten, SJ; Nomanbhoy, TK; Cravatt, BF; Ahn, K Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH Inhibitor. ACS Med Chem Lett2:91-96 (2011) [PubMed] Article