 Found 205 hits with Last Name = 'dudley' and Initial = 'dt'
Found 205 hits with Last Name = 'dudley' and Initial = 'dt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin A receptor in cultured rabbit renal artery vascular smooth muscle cells |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin B receptor in cultured rat cerebellar membranes |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25077
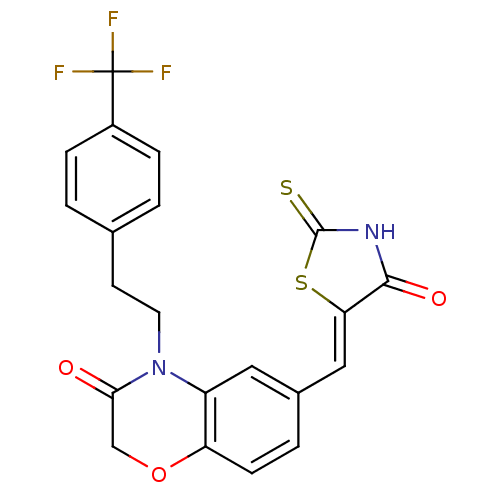
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES FC(F)(F)c1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H15F3N2O3S2/c22-21(23,24)14-4-1-12(2-5-14)7-8-26-15-9-13(3-6-16(15)29-11-18(26)27)10-17-19(28)25-20(30)31-17/h1-6,9-10H,7-8,11H2,(H,25,28,30)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25073
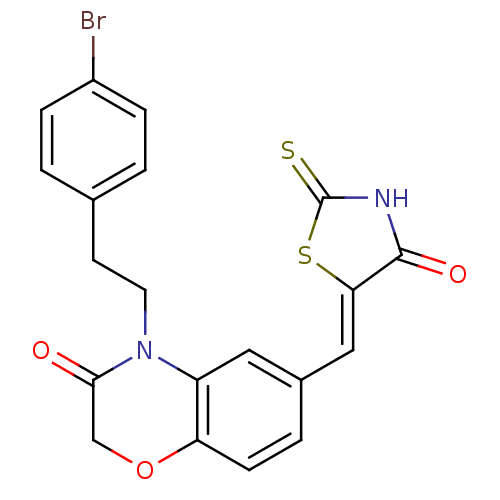
(4-[2-(4-bromophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Brc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15BrN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25061
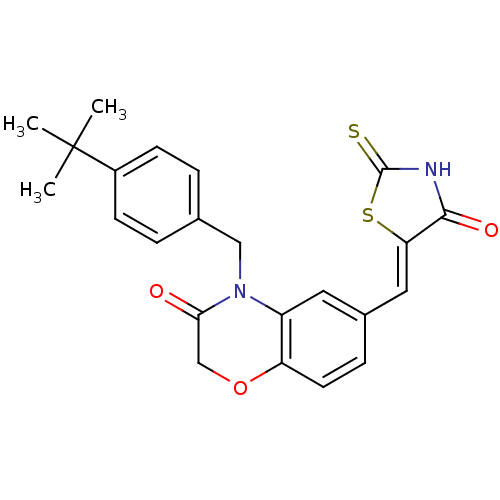
(4-[(4-tert-butylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES CC(C)(C)c1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C23H22N2O3S2/c1-23(2,3)16-7-4-14(5-8-16)12-25-17-10-15(6-9-18(17)28-13-20(25)26)11-19-21(27)24-22(29)30-19/h4-11H,12-13H2,1-3H3,(H,24,27,29)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25066
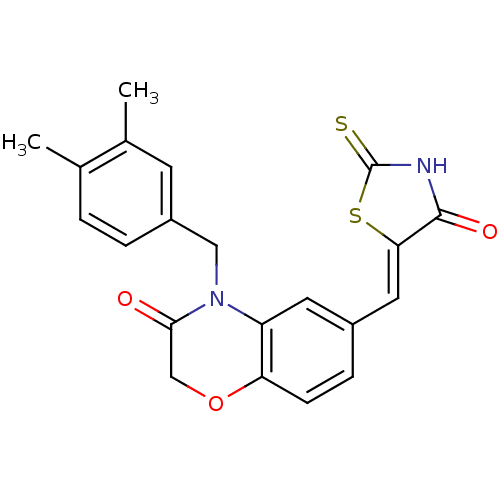
(4-[(3,4-dimethylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES Cc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1C Show InChI InChI=1S/C21H18N2O3S2/c1-12-3-4-15(7-13(12)2)10-23-16-8-14(5-6-17(16)26-11-19(23)24)9-18-20(25)22-21(27)28-18/h3-9H,10-11H2,1-2H3,(H,22,25,27)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25068
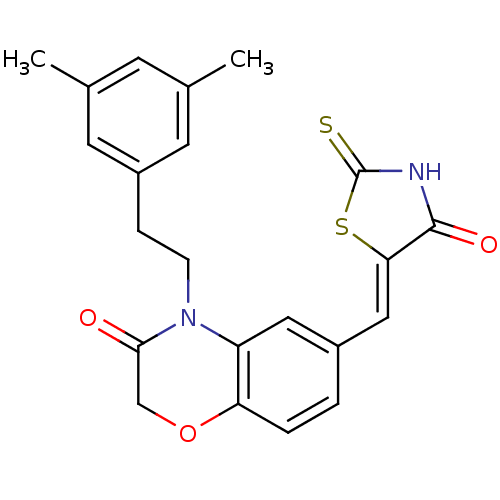
(4-[2-(3,5-dimethylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Cc1cc(C)cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C22H20N2O3S2/c1-13-7-14(2)9-16(8-13)5-6-24-17-10-15(3-4-18(17)27-12-20(24)25)11-19-21(26)23-22(28)29-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,28)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25080
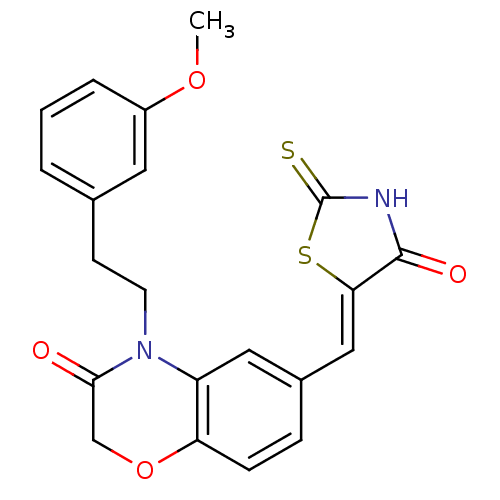
(4-[2-(3-methoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulf...)Show SMILES COc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O4S2/c1-26-15-4-2-3-13(9-15)7-8-23-16-10-14(5-6-17(16)27-12-19(23)24)11-18-20(25)22-21(28)29-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,28)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25069
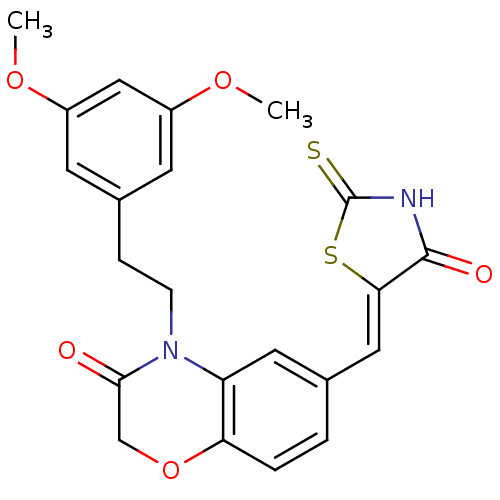
(4-[2-(3,5-dimethoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-...)Show SMILES COc1cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc(OC)c1 Show InChI InChI=1S/C22H20N2O5S2/c1-27-15-7-14(8-16(11-15)28-2)5-6-24-17-9-13(3-4-18(17)29-12-20(24)25)10-19-21(26)23-22(30)31-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,30)/b19-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25074
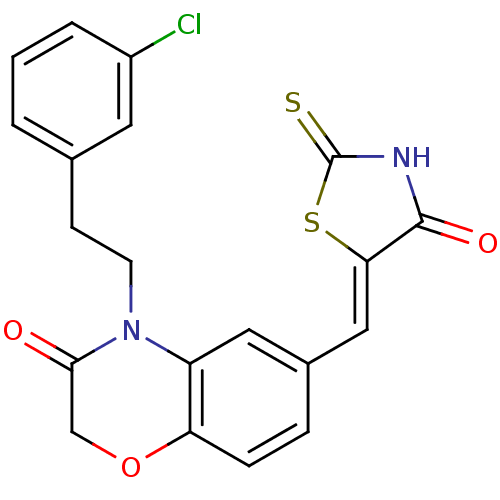
(4-[2-(3-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-3-1-2-12(8-14)6-7-23-15-9-13(4-5-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-5,8-10H,6-7,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.27 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25078
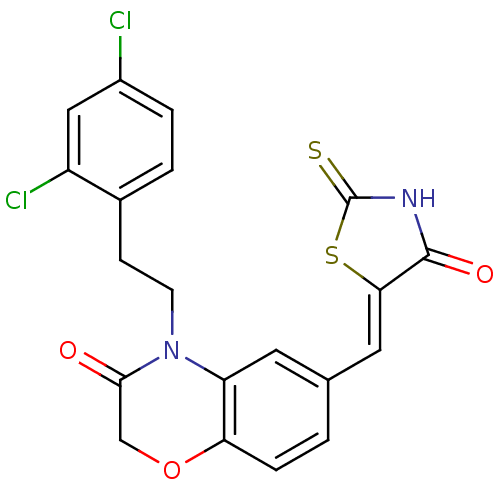
(4-[2-(2,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c(Cl)c1 Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-2-12(14(22)9-13)5-6-24-15-7-11(1-4-16(15)27-10-18(24)25)8-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.28 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25075
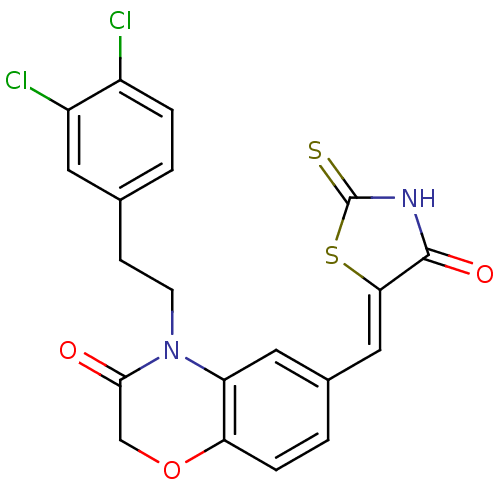
(4-[2-(3,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1Cl Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.36 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25070
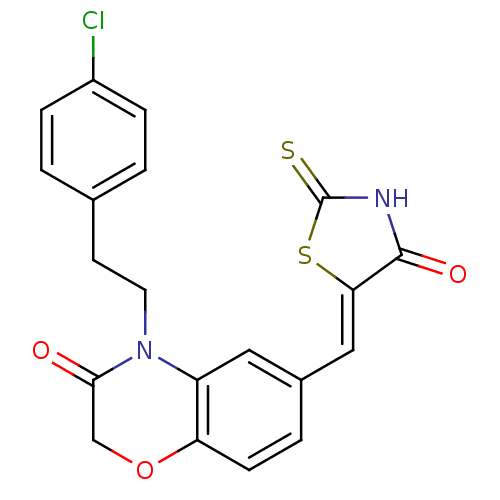
(4-[2-(4-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.76 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25081
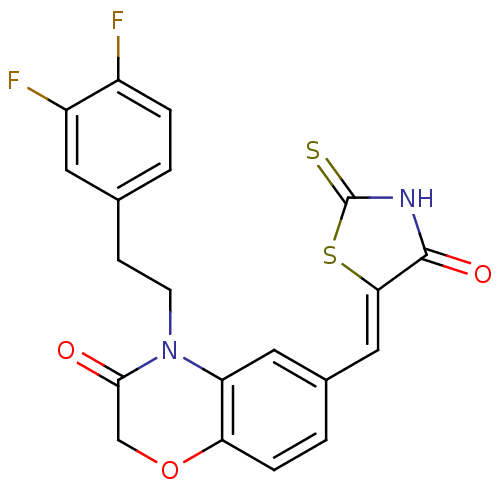
(4-[2-(3,4-difluorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Fc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1F Show InChI InChI=1S/C20H14F2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.84 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25079
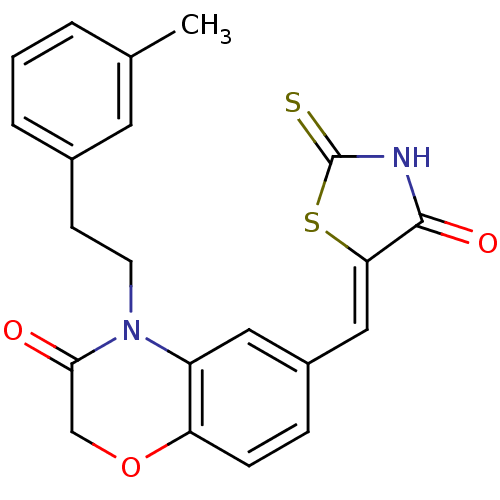
(4-[2-(3-methylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Cc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O3S2/c1-13-3-2-4-14(9-13)7-8-23-16-10-15(5-6-17(16)26-12-19(23)24)11-18-20(25)22-21(27)28-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,27)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.96 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25071
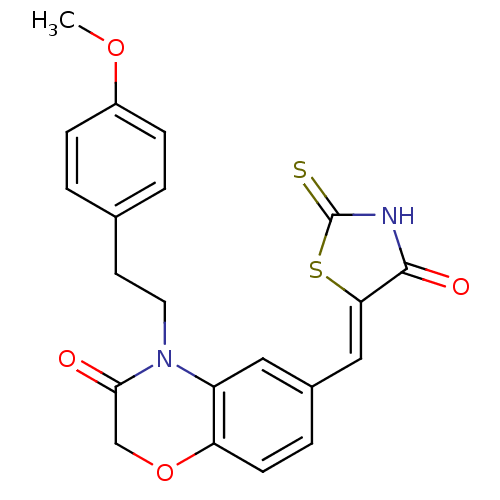
(4-[2-(4-methoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulf...)Show SMILES COc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H18N2O4S2/c1-26-15-5-2-13(3-6-15)8-9-23-16-10-14(4-7-17(16)27-12-19(23)24)11-18-20(25)22-21(28)29-18/h2-7,10-11H,8-9,12H2,1H3,(H,22,25,28)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.57 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25062
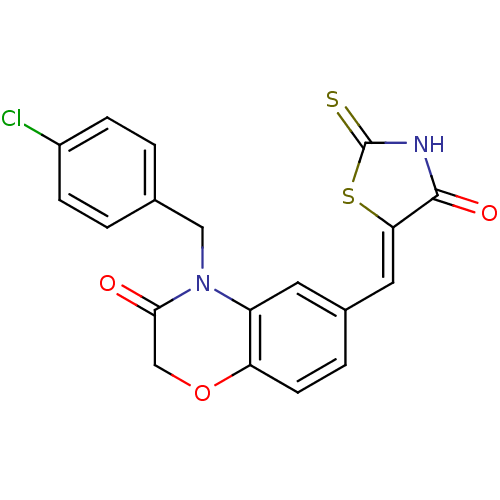
(4-[(4-chlorophenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Clc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C19H13ClN2O3S2/c20-13-4-1-11(2-5-13)9-22-14-7-12(3-6-15(14)25-10-17(22)23)8-16-18(24)21-19(26)27-16/h1-8H,9-10H2,(H,21,24,26)/b16-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.16 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50335377

(CHEMBL1651534 | N-Pyridazin-3-yl-4-(3-{[5-(trifluo...)Show SMILES FC(F)(F)c1ccc(-[#8]-c2cccc(\[#6]=[#6]-3\[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-[#7]-c3cccnn3)c2)nc1 Show InChI InChI=1S/C23H20F3N5O2/c24-23(25,26)18-6-7-21(27-15-18)33-19-4-1-3-17(14-19)13-16-8-11-31(12-9-16)22(32)29-20-5-2-10-28-30-20/h1-7,10,13-15H,8-9,11-12H2,(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human FAAH N-terminal transmembrane-deleted truncated form expressed in Escherichia coli preincubated for 60 mins before ole... |
ACS Med Chem Lett 2: 91-96 (2011)
Article DOI: 10.1021/ml100190t
BindingDB Entry DOI: 10.7270/Q25X29WM |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50335377

(CHEMBL1651534 | N-Pyridazin-3-yl-4-(3-{[5-(trifluo...)Show SMILES FC(F)(F)c1ccc(-[#8]-c2cccc(\[#6]=[#6]-3\[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-[#7]-c3cccnn3)c2)nc1 Show InChI InChI=1S/C23H20F3N5O2/c24-23(25,26)18-6-7-21(27-15-18)33-19-4-1-3-17(14-19)13-16-8-11-31(12-9-16)22(32)29-20-5-2-10-28-30-20/h1-7,10,13-15H,8-9,11-12H2,(H,29,30,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of His-tagged rat FAAH N-terminal transmembrane-deleted truncated form expressed in Escherichia coli preincubated for 60 mins... |
ACS Med Chem Lett 2: 91-96 (2011)
Article DOI: 10.1021/ml100190t
BindingDB Entry DOI: 10.7270/Q25X29WM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25072
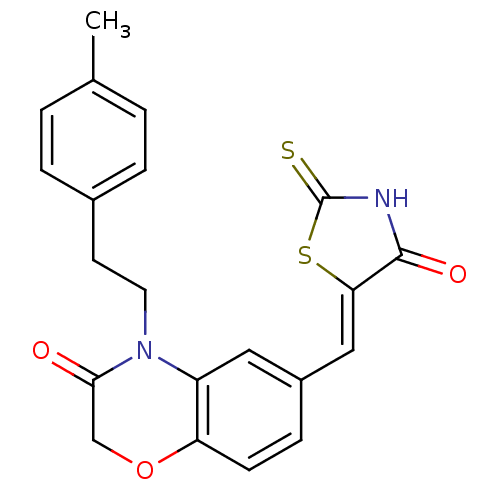
(4-[2-(4-methylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Cc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H18N2O3S2/c1-13-2-4-14(5-3-13)8-9-23-16-10-15(6-7-17(16)26-12-19(23)24)11-18-20(25)22-21(27)28-18/h2-7,10-11H,8-9,12H2,1H3,(H,22,25,27)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.83 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50032172

((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin A receptor in cultured rabbit renal artery vascular smooth muscle cells |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM15234
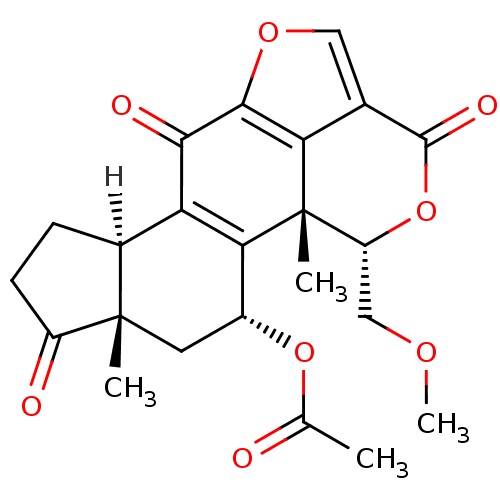
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25059
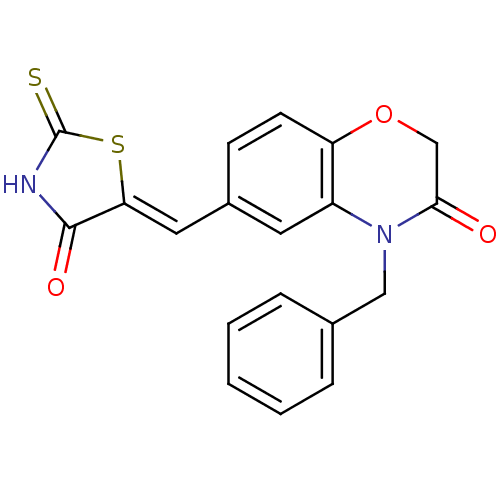
(4-benzyl-6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazo...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(Cc3ccccc3)c2c1 Show InChI InChI=1S/C19H14N2O3S2/c22-17-11-24-15-7-6-13(9-16-18(23)20-19(25)26-16)8-14(15)21(17)10-12-4-2-1-3-5-12/h1-9H,10-11H2,(H,20,23,25)/b16-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25067
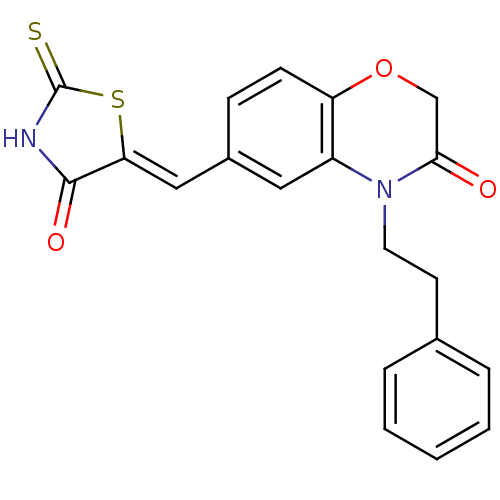
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(CCc3ccccc3)c2c1 Show InChI InChI=1S/C20H16N2O3S2/c23-18-12-25-16-7-6-14(11-17-19(24)21-20(26)27-17)10-15(16)22(18)9-8-13-4-2-1-3-5-13/h1-7,10-11H,8-9,12H2,(H,21,24,26)/b17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B/1 receptor
(RAT) | BDBM50045239
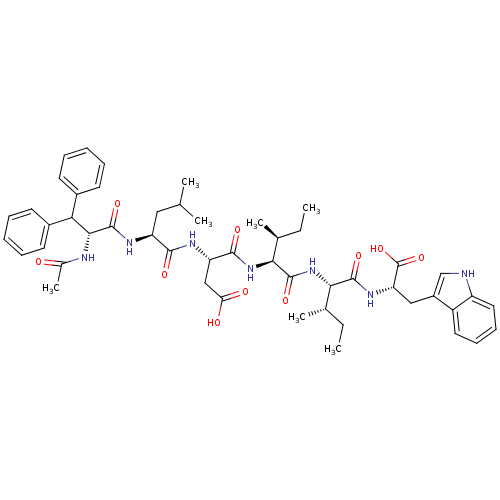
(Ac-D-Dip-Leu-Asp-Ile-Ile-Trp | Ac-D-Dip-Leu-Asp-Il...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Endothelin receptor from rat heart ventricle |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25064
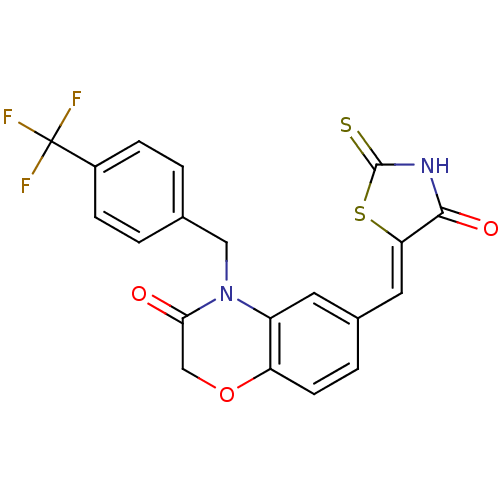
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES FC(F)(F)c1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H13F3N2O3S2/c21-20(22,23)13-4-1-11(2-5-13)9-25-14-7-12(3-6-15(14)28-10-17(25)26)8-16-18(27)24-19(29)30-16/h1-8H,9-10H2,(H,24,27,29)/b16-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27.5 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25060
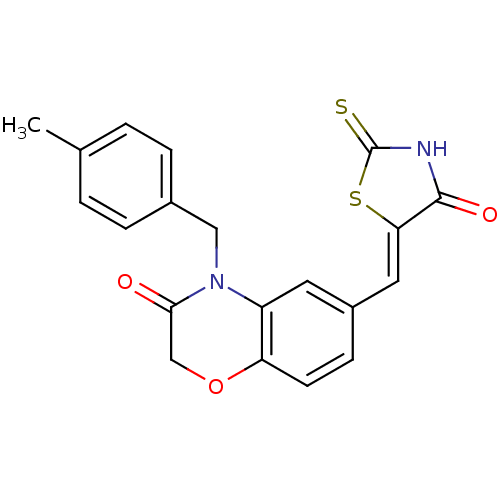
(4-[(4-methylphenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Cc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H16N2O3S2/c1-12-2-4-13(5-3-12)10-22-15-8-14(6-7-16(15)25-11-18(22)23)9-17-19(24)21-20(26)27-17/h2-9H,10-11H2,1H3,(H,21,24,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25076
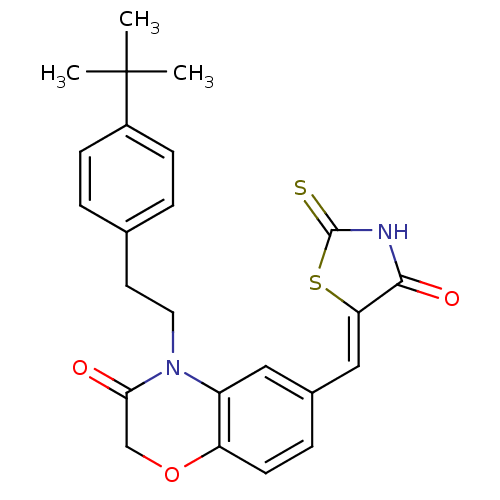
(4-[2-(4-tert-butylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES CC(C)(C)c1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C24H24N2O3S2/c1-24(2,3)17-7-4-15(5-8-17)10-11-26-18-12-16(6-9-19(18)29-14-21(26)27)13-20-22(28)25-23(30)31-20/h4-9,12-13H,10-11,14H2,1-3H3,(H,25,28,30)/b20-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33.2 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50045240
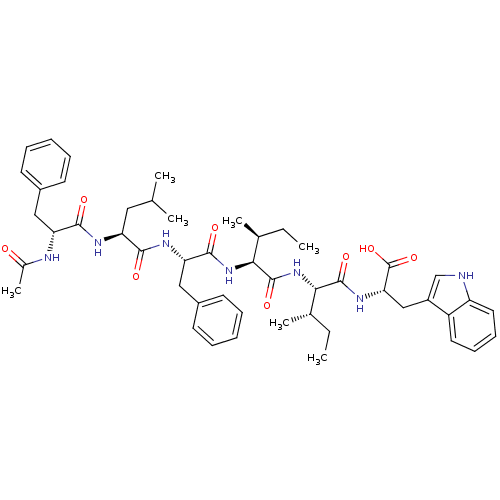
((S)-2-[(S)-2-((2S,5S)-2-{(S)-2-[(S)-2-((R)-2-Acety...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C49H65N7O8/c1-8-30(5)42(47(61)54-41(49(63)64)27-35-28-50-37-23-17-16-22-36(35)37)56-48(62)43(31(6)9-2)55-46(60)40(26-34-20-14-11-15-21-34)53-44(58)38(24-29(3)4)52-45(59)39(51-32(7)57)25-33-18-12-10-13-19-33/h10-23,28-31,38-43,50H,8-9,24-27H2,1-7H3,(H,51,57)(H,52,59)(H,53,58)(H,54,61)(H,55,60)(H,56,62)(H,63,64)/t30-,31-,38-,39+,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25063
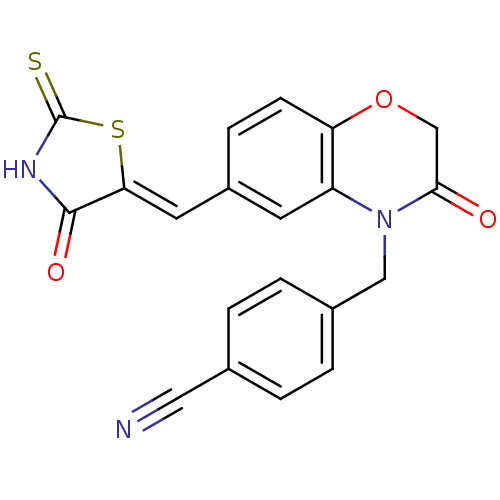
(4-[(3-oxo-6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiaz...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(Cc3ccc(cc3)C#N)c2c1 Show InChI InChI=1S/C20H13N3O3S2/c21-9-12-1-3-13(4-2-12)10-23-15-7-14(5-6-16(15)26-11-18(23)24)8-17-19(25)22-20(27)28-17/h1-8H,10-11H2,(H,22,25,27)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36.1 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B/1 receptor
(RAT) | BDBM50368647
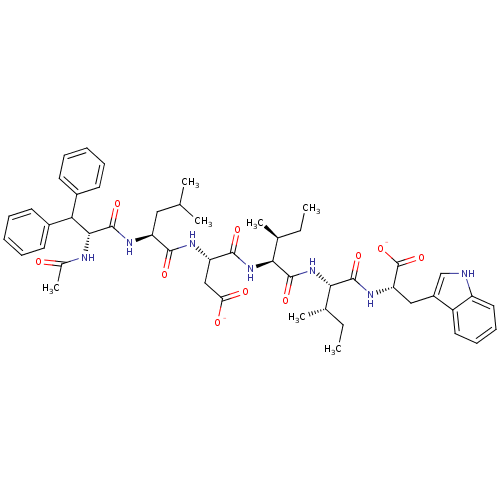
(CHEMBL1788127)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC([O-])=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C([O-])=O |r| Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/p-2/t29-,30-,37-,38-,39-,42-,43-,44+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Endothelin receptor from rat heart ventricle |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50008064
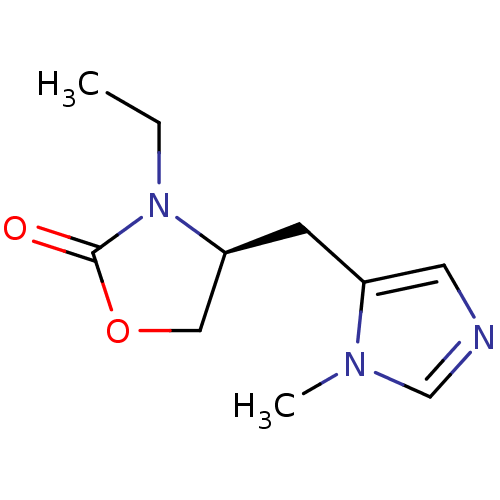
((S)-3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-ox...)Show InChI InChI=1S/C10H15N3O2/c1-3-13-9(6-15-10(13)14)4-8-5-11-7-12(8)2/h5,7,9H,3-4,6H2,1-2H3/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace muscarinic agonist [3H]quinuclidinyl benzilate in rat cortical tissue (RCMD binding assay), at 0.1... |
Bioorg Med Chem Lett 1: 147-150 (1991)
Article DOI: 10.1016/S0960-894X(01)80787-3
BindingDB Entry DOI: 10.7270/Q2513Z3K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25065
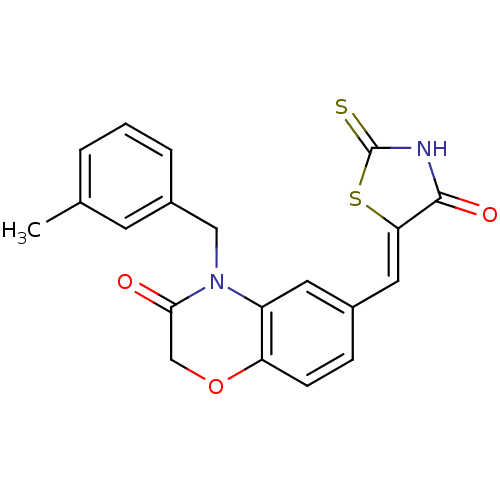
(4-[(3-methylphenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Cc1cccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C20H16N2O3S2/c1-12-3-2-4-14(7-12)10-22-15-8-13(5-6-16(15)25-11-18(22)23)9-17-19(24)21-20(26)27-17/h2-9H,10-11H2,1H3,(H,21,24,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50284007
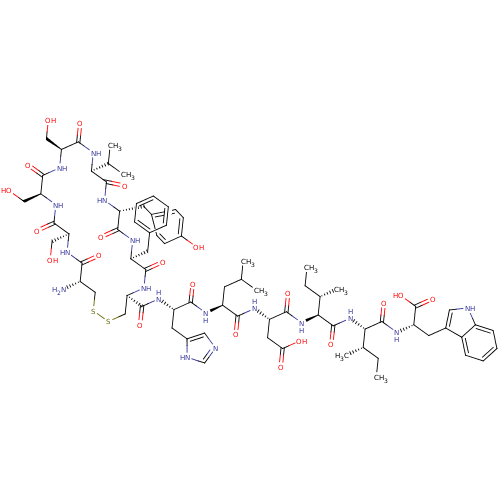
(CHEMBL412460 | Trp-Ile-Ile-Asp-Leu-Hisc(Cys-Ser-Se...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CSSC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C77H107N17O21S2/c1-9-40(7)62(75(112)87-55(77(114)115)27-44-30-80-49-19-15-14-18-47(44)49)94-76(113)63(41(8)10-2)93-69(106)54(29-60(99)100)85-65(102)50(24-38(3)4)82-68(105)53(28-45-31-79-37-81-45)84-73(110)59-36-117-116-35-48(78)64(101)88-56(32-95)70(107)89-57(33-96)71(108)90-58(34-97)72(109)92-61(39(5)6)74(111)86-52(26-43-20-22-46(98)23-21-43)66(103)83-51(67(104)91-59)25-42-16-12-11-13-17-42/h11-23,30-31,37-41,48,50-59,61-63,80,95-98H,9-10,24-29,32-36,78H2,1-8H3,(H,79,81)(H,82,105)(H,83,103)(H,84,110)(H,85,102)(H,86,111)(H,87,112)(H,88,101)(H,89,107)(H,90,108)(H,91,104)(H,92,109)(H,93,106)(H,94,113)(H,99,100)(H,114,115)/t40-,41-,48-,50-,51-,52+,53-,54-,55-,56-,57-,58-,59+,61-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Endothelin B receptor in rat cerebellar membrane (ET-B) from adult blue laurie rat |
Bioorg Med Chem Lett 4: 567-572 (1994)
Article DOI: 10.1016/S0960-894X(01)80156-6
BindingDB Entry DOI: 10.7270/Q2TT4QW6 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50045244

((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C47H59N7O10/c1-6-27(3)40(45(61)52-38(47(63)64)24-32-26-48-34-21-15-14-20-33(32)34)54-46(62)41(28(4)7-2)53-44(60)37(25-39(56)57)51-43(59)36(23-31-18-12-9-13-19-31)50-42(58)35(49-29(5)55)22-30-16-10-8-11-17-30/h8-21,26-28,35-38,40-41,48H,6-7,22-25H2,1-5H3,(H,49,55)(H,50,58)(H,51,59)(H,52,61)(H,53,60)(H,54,62)(H,56,57)(H,63,64)/t27-,28-,35+,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50368647

(CHEMBL1788127)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC([O-])=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C([O-])=O |r| Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/p-2/t29-,30-,37-,38-,39-,42-,43-,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against ET A receptor from rabbit renal artery vascular smooth muscle cells |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50045244

((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C47H59N7O10/c1-6-27(3)40(45(61)52-38(47(63)64)24-32-26-48-34-21-15-14-20-33(32)34)54-46(62)41(28(4)7-2)53-44(60)37(25-39(56)57)51-43(59)36(23-31-18-12-9-13-19-31)50-42(58)35(49-29(5)55)22-30-16-10-8-11-17-30/h8-21,26-28,35-38,40-41,48H,6-7,22-25H2,1-5H3,(H,49,55)(H,50,58)(H,51,59)(H,52,61)(H,53,60)(H,54,62)(H,56,57)(H,63,64)/t27-,28-,35+,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368647

(CHEMBL1788127)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC([O-])=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C([O-])=O |r| Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/p-2/t29-,30-,37-,38-,39-,42-,43-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50045239

(Ac-D-Dip-Leu-Asp-Ile-Ile-Trp | Ac-D-Dip-Leu-Asp-Il...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25058
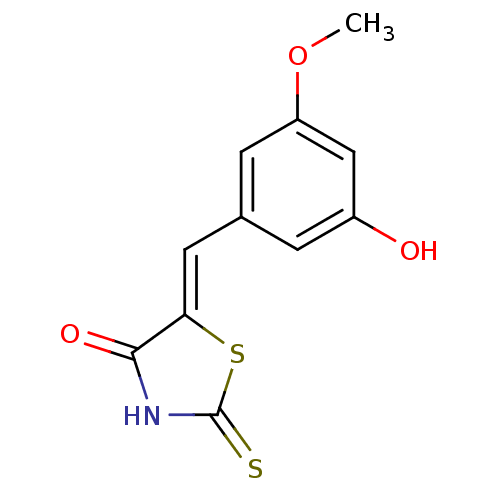
((5Z)-5-[(3-hydroxy-5-methoxyphenyl)methylidene]-2-...)Show InChI InChI=1S/C11H9NO3S2/c1-15-8-3-6(2-7(13)5-8)4-9-10(14)12-11(16)17-9/h2-5,13H,1H3,(H,12,14,16)/b9-4- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77.7 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50001220

(3-{2-[2-Acetylamino-3-(1H-indol-3-yl)-propionylami...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C46H62N8O10/c1-8-25(5)39(44(61)52-37(46(63)64)20-29-23-48-33-17-13-11-15-31(29)33)54-45(62)40(26(6)9-2)53-43(60)36(21-38(56)57)51-41(58)34(18-24(3)4)50-42(59)35(49-27(7)55)19-28-22-47-32-16-12-10-14-30(28)32/h10-17,22-26,34-37,39-40,47-48H,8-9,18-21H2,1-7H3,(H,49,55)(H,50,59)(H,51,58)(H,52,61)(H,53,60)(H,54,62)(H,56,57)(H,63,64)/t25-,26-,34-,35+,36-,37-,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin B receptor in cultured rat cerebellar membranes |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B/1 receptor
(RAT) | BDBM50045230

(Ac-D-Trp-Leu-Asp-Ile-Ile-Trp | CHEMBL313382)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C46H62N8O10/c1-8-25(5)39(44(61)52-37(46(63)64)20-29-23-48-33-17-13-11-15-31(29)33)54-45(62)40(26(6)9-2)53-43(60)36(21-38(56)57)51-41(58)34(18-24(3)4)50-42(59)35(49-27(7)55)19-28-22-47-32-16-12-10-14-30(28)32/h10-17,22-26,34-37,39-40,47-48H,8-9,18-21H2,1-7H3,(H,49,55)(H,50,59)(H,51,58)(H,52,61)(H,53,60)(H,54,62)(H,56,57)(H,63,64)/t25-,26-,34-,35-,36-,37-,39-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Endothelin receptor from rat heart ventricle |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50001220

(3-{2-[2-Acetylamino-3-(1H-indol-3-yl)-propionylami...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C46H62N8O10/c1-8-25(5)39(44(61)52-37(46(63)64)20-29-23-48-33-17-13-11-15-31(29)33)54-45(62)40(26(6)9-2)53-43(60)36(21-38(56)57)51-41(58)34(18-24(3)4)50-42(59)35(49-27(7)55)19-28-22-47-32-16-12-10-14-30(28)32/h10-17,22-26,34-37,39-40,47-48H,8-9,18-21H2,1-7H3,(H,49,55)(H,50,59)(H,51,58)(H,52,61)(H,53,60)(H,54,62)(H,56,57)(H,63,64)/t25-,26-,34-,35+,36-,37-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin A receptor in cultured rabbit renal artery vascular smooth muscle cells |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50045239

(Ac-D-Dip-Leu-Asp-Ile-Ile-Trp | Ac-D-Dip-Leu-Asp-Il...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against ET A receptor from rabbit renal artery vascular smooth muscle cells |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50032172

((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin B receptor in cultured rat cerebellar membranes |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50045257
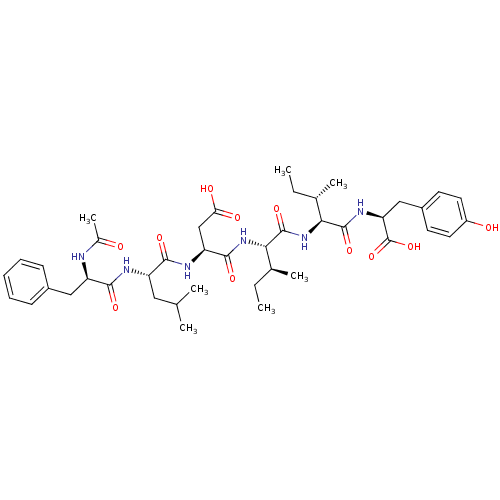
(Ac-D-Phe-Leu-Asp-Ile-Ile-Tyr | CHEMBL89736)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C42H60N6O11/c1-8-24(5)35(40(56)46-33(42(58)59)21-28-15-17-29(50)18-16-28)48-41(57)36(25(6)9-2)47-39(55)32(22-34(51)52)45-37(53)30(19-23(3)4)44-38(54)31(43-26(7)49)20-27-13-11-10-12-14-27/h10-18,23-25,30-33,35-36,50H,8-9,19-22H2,1-7H3,(H,43,49)(H,44,54)(H,45,53)(H,46,56)(H,47,55)(H,48,57)(H,51,52)(H,58,59)/t24-,25-,30-,31+,32-,33-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50045244

((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C47H59N7O10/c1-6-27(3)40(45(61)52-38(47(63)64)24-32-26-48-34-21-15-14-20-33(32)34)54-46(62)41(28(4)7-2)53-44(60)37(25-39(56)57)51-43(59)36(23-31-18-12-9-13-19-31)50-42(58)35(49-29(5)55)22-30-16-10-8-11-17-30/h8-21,26-28,35-38,40-41,48H,6-7,22-25H2,1-5H3,(H,49,55)(H,50,58)(H,51,59)(H,52,61)(H,53,60)(H,54,62)(H,56,57)(H,63,64)/t27-,28-,35+,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against ET A receptor from rabbit renal artery vascular smooth muscle cells |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50045244

((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C47H59N7O10/c1-6-27(3)40(45(61)52-38(47(63)64)24-32-26-48-34-21-15-14-20-33(32)34)54-46(62)41(28(4)7-2)53-44(60)37(25-39(56)57)51-43(59)36(23-31-18-12-9-13-19-31)50-42(58)35(49-29(5)55)22-30-16-10-8-11-17-30/h8-21,26-28,35-38,40-41,48H,6-7,22-25H2,1-5H3,(H,49,55)(H,50,58)(H,51,59)(H,52,61)(H,53,60)(H,54,62)(H,56,57)(H,63,64)/t27-,28-,35+,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against ET A receptor from rabbit renal artery vascular smooth muscle cells |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50284006

(CHEMBL438380 | Trp-Ile-Ile-Asp-Leu-Hisc(Cys-Ser-Ao...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CSSC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCCCCCCCC(=O)N[C@H](C(C)C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C79H112N16O18S2/c1-9-45(7)66(77(110)90-60(79(112)113)34-49-37-83-54-24-19-18-23-52(49)54)95-78(111)67(46(8)10-2)94-74(107)59(36-64(99)100)88-70(103)55(31-43(3)4)85-73(106)58(35-50-38-81-42-84-50)87-75(108)62-41-115-114-40-53(80)68(101)91-61(39-96)69(102)82-30-20-13-11-12-17-25-63(98)93-65(44(5)6)76(109)89-57(33-48-26-28-51(97)29-27-48)71(104)86-56(72(105)92-62)32-47-21-15-14-16-22-47/h14-16,18-19,21-24,26-29,37-38,42-46,53,55-62,65-67,83,96-97H,9-13,17,20,25,30-36,39-41,80H2,1-8H3,(H,81,84)(H,82,102)(H,85,106)(H,86,104)(H,87,108)(H,88,103)(H,89,109)(H,90,110)(H,91,101)(H,92,105)(H,93,98)(H,94,107)(H,95,111)(H,99,100)(H,112,113)/t45-,46-,53-,55-,56+,57-,58-,59-,60-,61-,62+,65+,66-,67-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Endothelin B receptor in rat cerebellar membrane (ET-B) from adult blue laurie rat |
Bioorg Med Chem Lett 4: 567-572 (1994)
Article DOI: 10.1016/S0960-894X(01)80156-6
BindingDB Entry DOI: 10.7270/Q2TT4QW6 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50001219
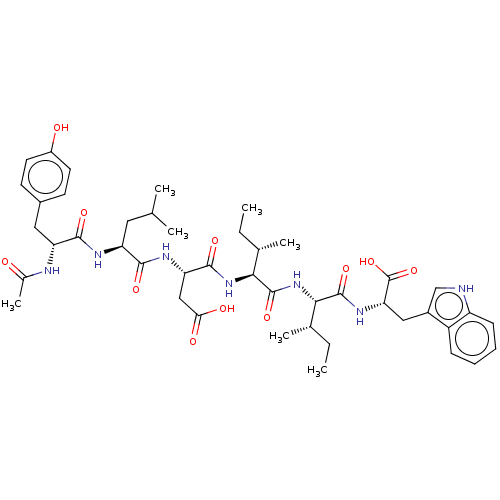
(3-{2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propionyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C44H61N7O11/c1-8-24(5)37(42(59)49-35(44(61)62)20-28-22-45-31-13-11-10-12-30(28)31)51-43(60)38(25(6)9-2)50-41(58)34(21-36(54)55)48-39(56)32(18-23(3)4)47-40(57)33(46-26(7)52)19-27-14-16-29(53)17-15-27/h10-17,22-25,32-35,37-38,45,53H,8-9,18-21H2,1-7H3,(H,46,52)(H,47,57)(H,48,56)(H,49,59)(H,50,58)(H,51,60)(H,54,55)(H,61,62)/t24-,25-,32-,33+,34-,35-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Effective concentration against Endothelin B receptor from rat cerebellum |
J Med Chem 36: 2585-94 (1993)
BindingDB Entry DOI: 10.7270/Q2V69K6K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data