

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Lysine-specific histone demethylase 1A | ||
| Ligand | BDBM105600 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Biochemical Assay | ||
| IC50 | <50±n/a nM | ||
| Citation |  Wu, L; Konkol, LC; Lajkiewicz, N; Lu, L; Xu, M; Yao, W; Yu, Z; Zhang, C; He, C Imidazopyridines and imidazopyrazines as LSD1 inhibitors US Patent US10640503 Publication Date 5/5/2020 Wu, L; Konkol, LC; Lajkiewicz, N; Lu, L; Xu, M; Yao, W; Yu, Z; Zhang, C; He, C Imidazopyridines and imidazopyrazines as LSD1 inhibitors US Patent US10640503 Publication Date 5/5/2020 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Lysine-specific histone demethylase 1A | |||
| Name: | Lysine-specific histone demethylase 1A | ||
| Synonyms: | AOF2 | BRAF35-HDAC complex protein BHC110 | Flavin-containing amine oxidase domain-containing protein 2 | KDM1 | KDM1A | KDM1A_HUMAN | KIAA0601 | LSD1 | Lysine-specific demethylase 1 (LSD1) | Lysine-specific histone demethylase 1 | Lysine-specific histone demethylase 1 (LSD1) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 92901.01 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | O60341 | ||
| Residue: | 852 | ||
| Sequence: |
| ||
| BDBM105600 | |||
| n/a | |||
| Name | BDBM105600 | ||
| Synonyms: | US10047086, 113 | US10640503, Example 113 | US9695168, 113 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C27H24FN7O2 | ||
| Mol. Mass. | 497.5236 | ||
| SMILES | CN(C)[C@H]1CCN(C1)c1nc(-c2ccc(cc2)C#N)c(-c2cc3oc(=O)n(C)c3cc2F)n2cncc12 |r,wU:3.2,(5.05,5.51,;6.14,4.42,;7.63,4.82,;5.75,2.93,;6.65,1.69,;5.75,.44,;4.28,.92,;4.28,2.46,;2.95,.15,;1.61,.92,;.28,.15,;-1.05,.92,;-1.05,2.46,;-2.39,3.23,;-3.72,2.46,;-3.72,.92,;-2.39,.15,;-5.06,3.23,;-6.39,4,;.28,-1.39,;-1.05,-2.16,;-2.39,-1.39,;-3.72,-2.16,;-5.19,-1.69,;-6.09,-2.93,;-7.63,-2.93,;-5.19,-4.18,;-5.96,-5.51,;-3.72,-3.7,;-2.39,-4.47,;-1.05,-3.7,;.28,-4.47,;1.61,-2.16,;1.93,-3.67,;3.46,-3.83,;4.09,-2.42,;2.95,-1.39,)| | ||
| Structure | 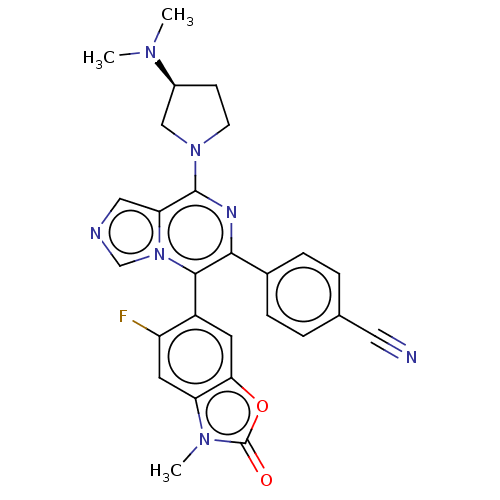
| ||