

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388434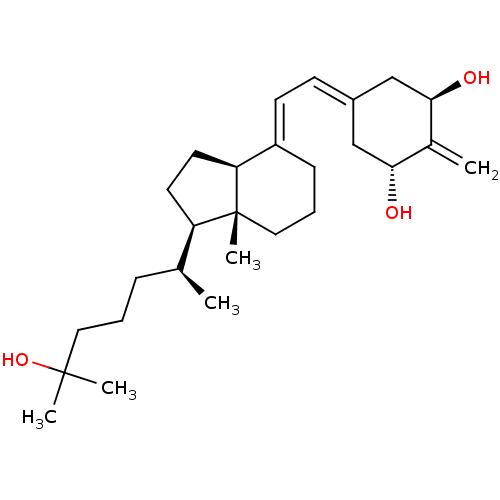 (CHEMBL605525) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417515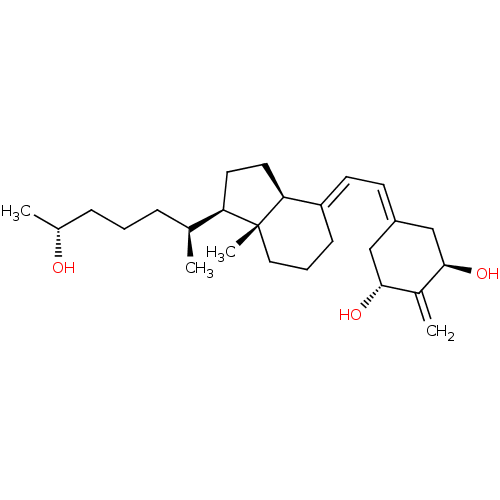 (CHEMBL1630755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073270 (CHEMBL333781 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073266 (CHEMBL119898 | {2-[(1S,3S,4S)-1-Benzyl-4-((2S,3S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135183 (CHEMBL3745798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50304585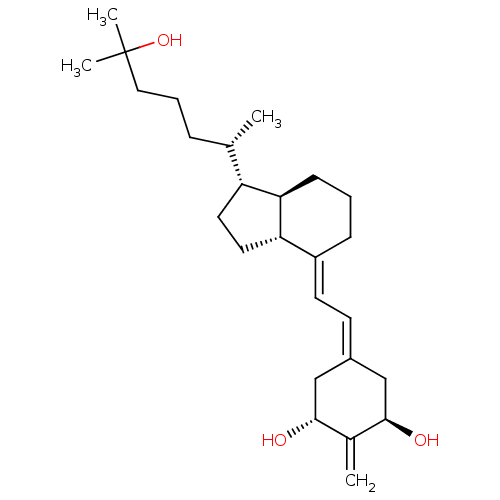 ((20S)-1alpha,25-Dihydroxy-2-methylene-18,19-dinorv...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of radiolabeled 1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 17: 7658-69 (2009) Article DOI: 10.1016/j.bmc.2009.09.047 BindingDB Entry DOI: 10.7270/Q26T0MQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388439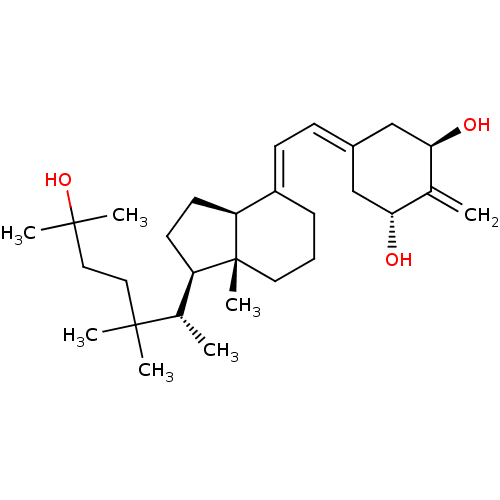 (CHEMBL2059272) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50068612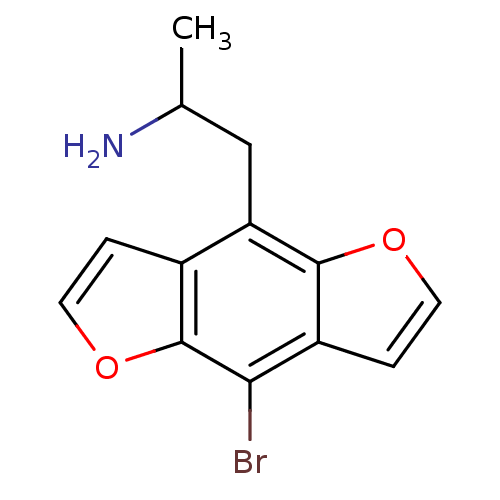 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417519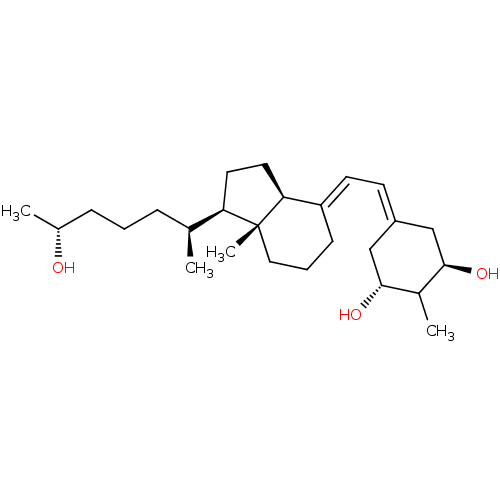 (CHEMBL1630759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,D531N,A572V] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <0.0210 | <-63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constant, Ki was determined by monitoring the competitive inhibition of the hydrolysis of the chromogenic substrate. | Biochemistry 42: 15029-35 (2003) Article DOI: 10.1021/bi035701y BindingDB Entry DOI: 10.7270/Q2V122ZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,A572V] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <0.0210 | <-63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constant, Ki was determined by monitoring the competitive inhibition of the hydrolysis of the chromogenic substrate. | Biochemistry 42: 15029-35 (2003) Article DOI: 10.1021/bi035701y BindingDB Entry DOI: 10.7270/Q2V122ZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0210 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constant, Ki was determined by monitoring the competitive inhibition of the hydrolysis of the chromogenic substrate. | Biochemistry 42: 15029-35 (2003) Article DOI: 10.1021/bi035701y BindingDB Entry DOI: 10.7270/Q2V122ZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50138568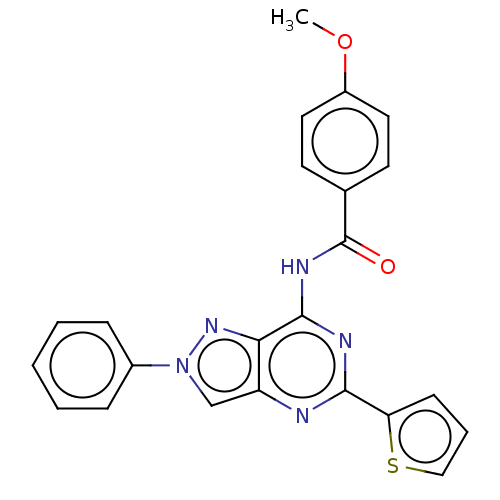 (CHEMBL3754229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA at human A3A receptor expressed in CHO cell membrane after 60 mins by scintillation counting method | Eur J Med Chem 108: 117-33 (2016) Article DOI: 10.1016/j.ejmech.2015.11.015 BindingDB Entry DOI: 10.7270/Q2NK3GWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,M537I,A572V] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constant, Ki was determined by monitoring the competitive inhibition of the hydrolysis of the chromogenic substrate. | Biochemistry 42: 15029-35 (2003) Article DOI: 10.1021/bi035701y BindingDB Entry DOI: 10.7270/Q2V122ZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135186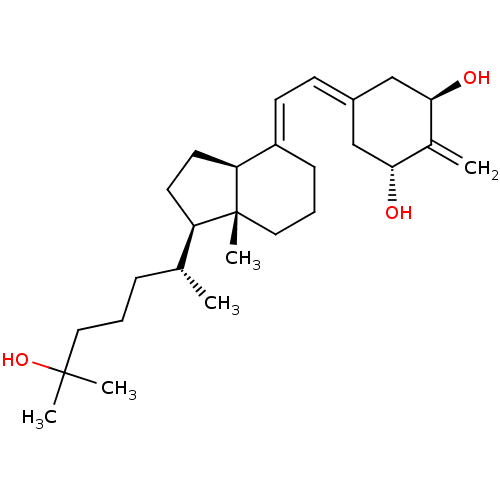 (CHEMBL1214620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135184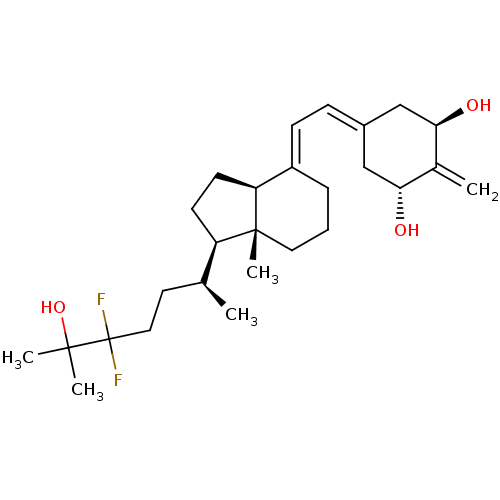 (CHEMBL3746058) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073253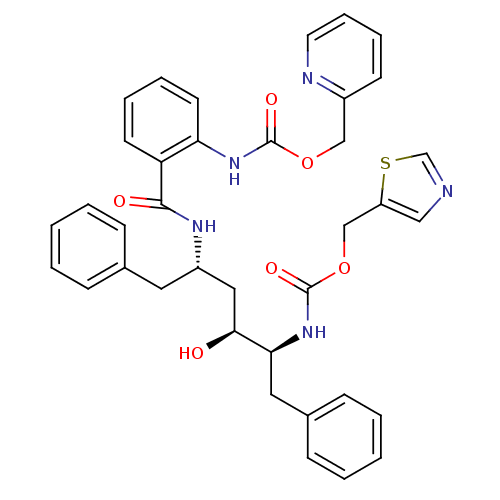 (CHEMBL278935 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135182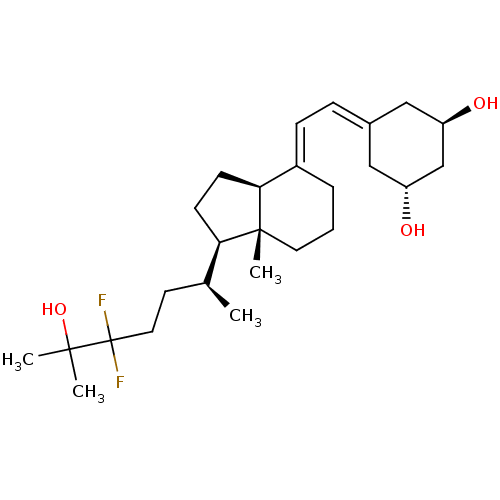 (CHEMBL3746107) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073250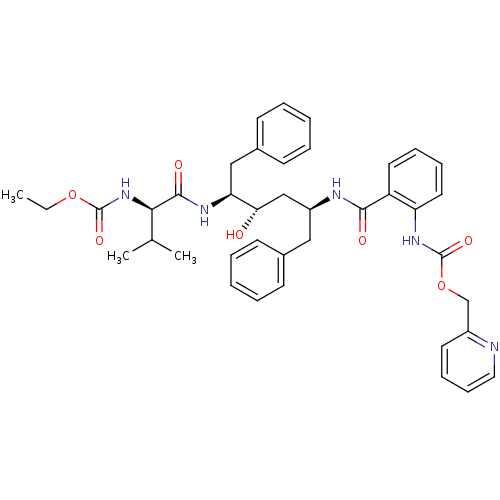 (CHEMBL333420 | {2-[(1S,3S,4S)-1-Benzyl-4-((R)-2-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135185 (CHEMBL3746759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073269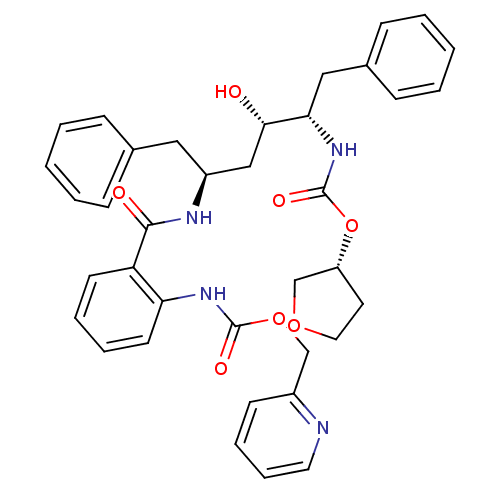 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-5-phenyl-4-[(R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388440 (CHEMBL2059269) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50563343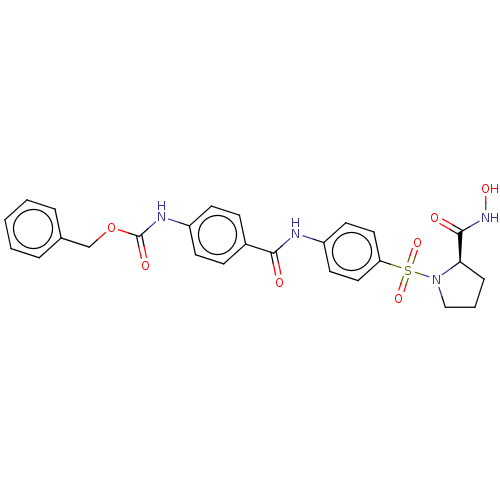 (CHEMBL4746390) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MMP2 (unknown origin) using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate incubated for 5 min followed by substrate addition and... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113260 BindingDB Entry DOI: 10.7270/Q2WW7NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904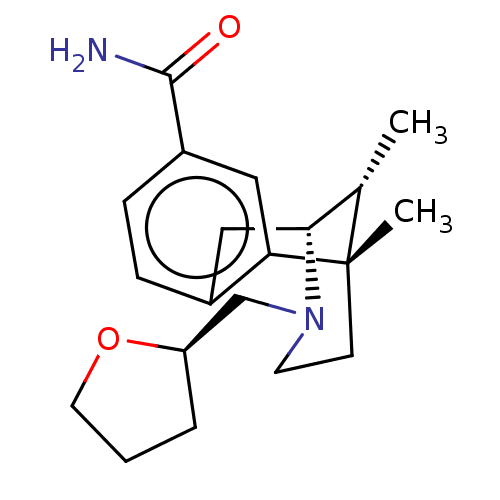 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | 58 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q2125W08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 16: 8563-73 (2008) Article DOI: 10.1016/j.bmc.2008.08.011 BindingDB Entry DOI: 10.7270/Q20001X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388438 (CHEMBL2059271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417520 (CHEMBL1630753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417520 (CHEMBL1630753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073252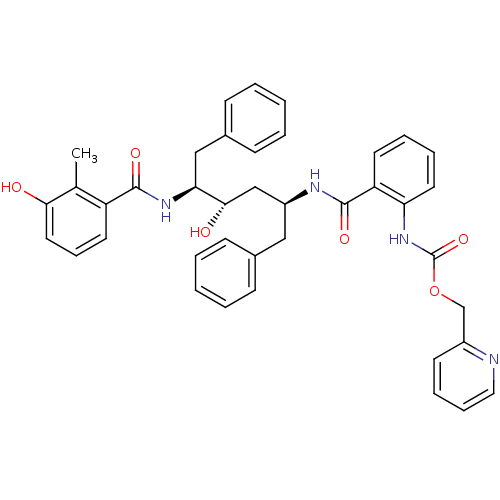 (CHEMBL324157 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254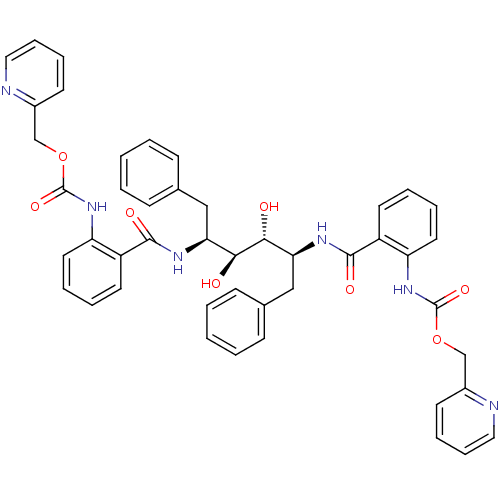 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM456904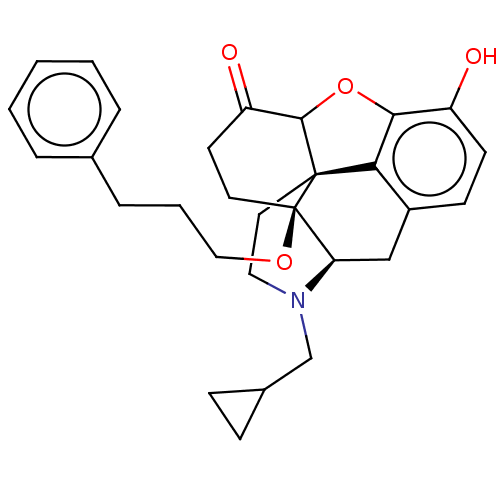 (US10736890, Compound TABLE B.14) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017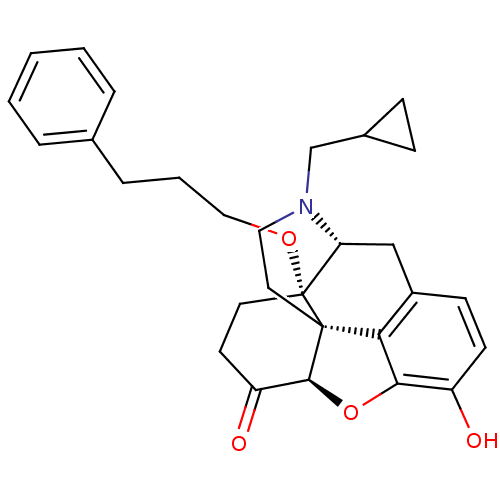 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285701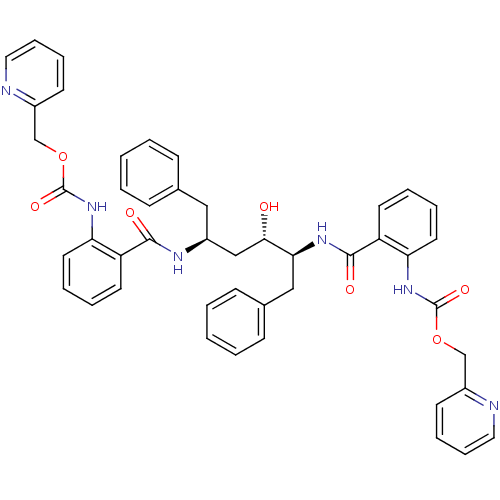 (Anthranilamide derivative | CHEMBL313767) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073265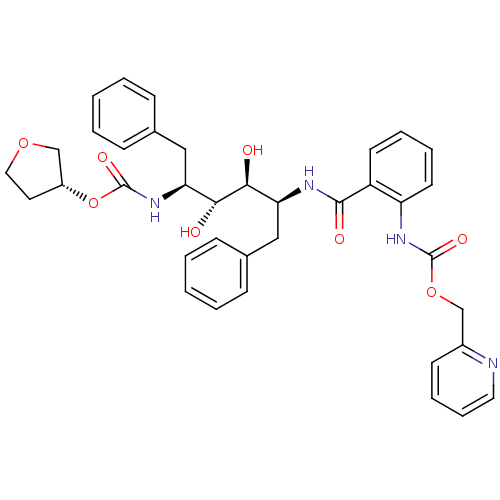 ((2-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-5-phenyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM456892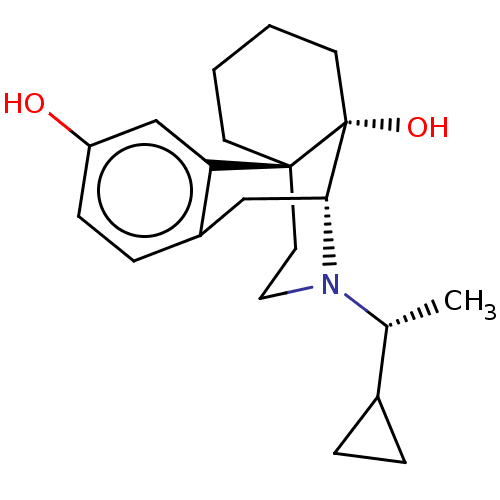 (US10736890, Compound TABLE B.2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 22387 total ) | Next | Last >> |