 Found 7206 hits with Last Name = 'yu' and Initial = 'z'
Found 7206 hits with Last Name = 'yu' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50086884
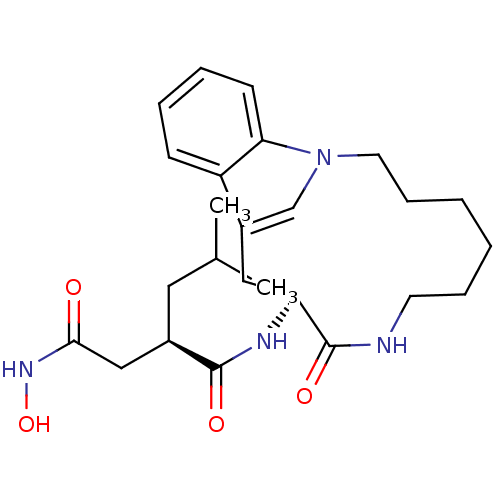
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C25H36N4O4/c1-17(2)13-18(15-23(30)28-33)24(31)27-21-14-19-16-29(22-10-6-5-9-20(19)22)12-8-4-3-7-11-26-25(21)32/h5-6,9-10,16-18,21,33H,3-4,7-8,11-15H2,1-2H3,(H,26,32)(H,27,31)(H,28,30)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human fibroblast collagenase, matrix metalloproteinase-1 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50086884

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C25H36N4O4/c1-17(2)13-18(15-23(30)28-33)24(31)27-21-14-19-16-29(22-10-6-5-9-20(19)22)12-8-4-3-7-11-26-25(21)32/h5-6,9-10,16-18,21,33H,3-4,7-8,11-15H2,1-2H3,(H,26,32)(H,27,31)(H,28,30)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil gelatinase, matrix metalloproteinase-2 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50410979
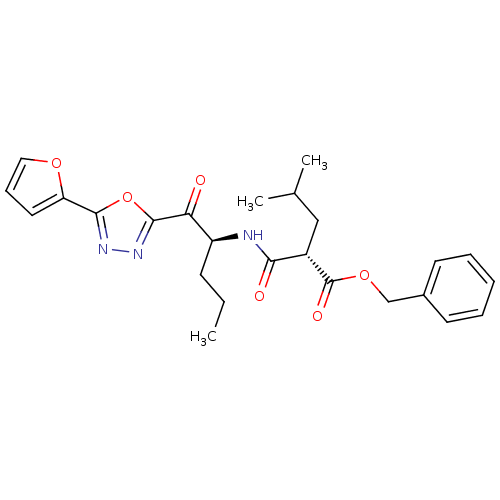
(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50410979

(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
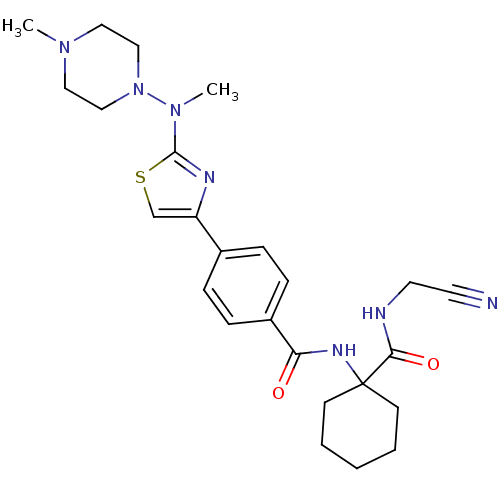
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50410971
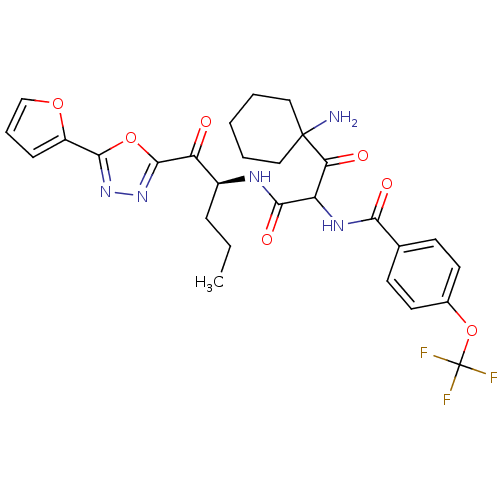
(CHEMBL378899)Show SMILES CCC[C@H](NC(=O)C(NC(=O)c1ccc(OC(F)(F)F)cc1)C(=O)C1(N)CCCCC1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C28H30F3N5O7/c1-2-7-18(21(37)26-36-35-25(42-26)19-8-6-15-41-19)33-24(40)20(22(38)27(32)13-4-3-5-14-27)34-23(39)16-9-11-17(12-10-16)43-28(29,30)31/h6,8-12,15,18,20H,2-5,7,13-14,32H2,1H3,(H,33,40)(H,34,39)/t18-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Competitive inhibition of PLK-4 (unknown origin) |
Eur J Med Chem 95: 35-40 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.020
BindingDB Entry DOI: 10.7270/Q2NG4SBV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50095768
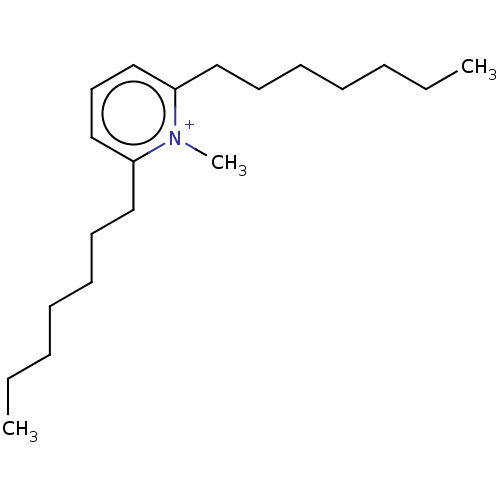
(CHEMBL3590463)Show SMILES [O-]S(=O)(=O)C(F)(F)F.CCCCCCCc1cccc(CCCCCCC)[n+]1C Show InChI InChI=1S/C20H36N.CHF3O3S/c1-4-6-8-10-12-15-19-17-14-18-20(21(19)3)16-13-11-9-7-5-2;2-1(3,4)8(5,6)7/h14,17-18H,4-13,15-16H2,1-3H3;(H,5,6,7)/q+1;/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human Kv11.1 expressed in HEK293 cell membranes by competitive radioligand displacement assay |
J Med Chem 58: 5916-29 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00518
BindingDB Entry DOI: 10.7270/Q2FX7C6V |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
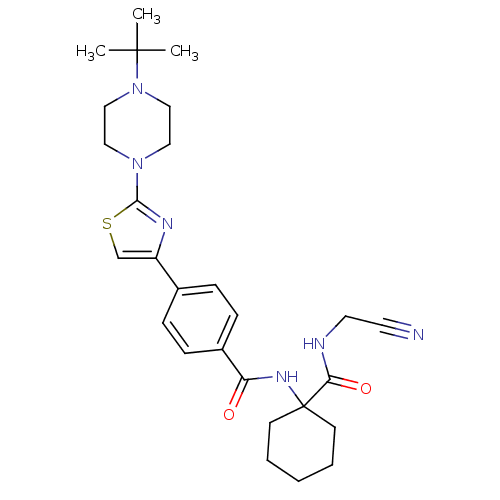
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50284753
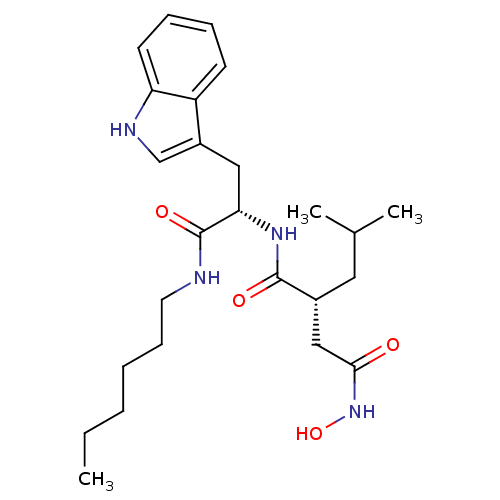
((R)-N*1*-[(S)-1-Hexylcarbamoyl-2-(1H-indol-3-yl)-e...)Show SMILES CCCCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C25H38N4O4/c1-4-5-6-9-12-26-25(32)22(14-19-16-27-21-11-8-7-10-20(19)21)28-24(31)18(13-17(2)3)15-23(30)29-33/h7-8,10-11,16-18,22,27,33H,4-6,9,12-15H2,1-3H3,(H,26,32)(H,28,31)(H,29,30)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil gelatinase, matrix metalloproteinase-2 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50531563
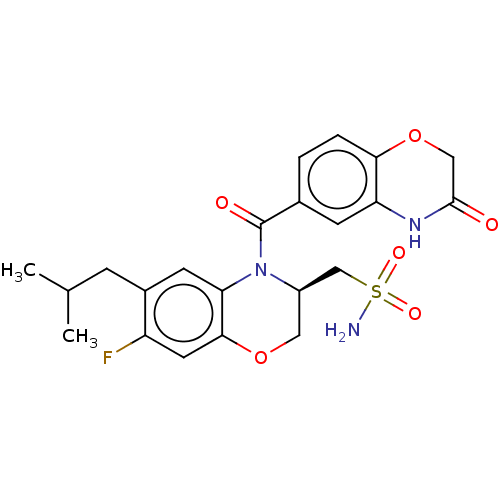
(CHEMBL4463240)Show SMILES CC(C)Cc1cc2N([C@@H](CS(N)(=O)=O)COc2cc1F)C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C22H24FN3O6S/c1-12(2)5-14-7-18-20(8-16(14)23)31-9-15(11-33(24,29)30)26(18)22(28)13-3-4-19-17(6-13)25-21(27)10-32-19/h3-4,6-8,12,15H,5,9-11H2,1-2H3,(H,25,27)(H2,24,29,30)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay |
J Med Chem 62: 1385-1406 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01523
BindingDB Entry DOI: 10.7270/Q2GT5RP6 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50531598

(CHEMBL4572476)Show SMILES CS(=O)(=O)C[C@H]1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C19H17FN2O6S/c1-29(25,26)10-13-8-27-17-7-12(20)3-4-15(17)22(13)19(24)11-2-5-16-14(6-11)21-18(23)9-28-16/h2-7,13H,8-10H2,1H3,(H,21,23)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay |
J Med Chem 62: 1385-1406 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01523
BindingDB Entry DOI: 10.7270/Q2GT5RP6 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50086884

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C25H36N4O4/c1-17(2)13-18(15-23(30)28-33)24(31)27-21-14-19-16-29(22-10-6-5-9-20(19)22)12-8-4-3-7-11-26-25(21)32/h5-6,9-10,16-18,21,33H,3-4,7-8,11-15H2,1-2H3,(H,26,32)(H,27,31)(H,28,30)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM89440
(4-(4-chlorophenyl)butyl-diethyl-heptyl-ammonium;to...)Show InChI InChI=1S/C21H37ClN/c1-4-7-8-9-11-18-23(5-2,6-3)19-12-10-13-20-14-16-21(22)17-15-20/h14-17H,4-13,18-19H2,1-3H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]astemizole from human ERG channel expressed in HEK293 cell membrane after 1 hr by scintillation counting analysis |
J Med Chem 56: 9427-40 (2013)
Article DOI: 10.1021/jm4010434
BindingDB Entry DOI: 10.7270/Q21C20T7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410609
(CHEMBL198798)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C29H38N6O2S/c30-15-16-31-27(37)29(13-3-1-4-14-29)33-26(36)23-9-7-22(8-10-23)25-21-38-28(32-25)35-19-11-24(12-20-35)34-17-5-2-6-18-34/h7-10,21,24H,1-6,11-14,16-20H2,(H,31,37)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410590
(CHEMBL200543)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCN(CC1)C1CCOCC1 Show InChI InChI=1S/C28H36N6O3S/c29-12-13-30-26(36)28(10-2-1-3-11-28)32-25(35)22-6-4-21(5-7-22)24-20-38-27(31-24)34-16-14-33(15-17-34)23-8-18-37-19-9-23/h4-7,20,23H,1-3,8-11,13-19H2,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410587
(CHEMBL200602)Show SMILES COCCN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H34N6O3S/c1-35-18-17-31-13-15-32(16-14-31)25-29-22(19-36-25)20-5-7-21(8-6-20)23(33)30-26(9-3-2-4-10-26)24(34)28-12-11-27/h5-8,19H,2-4,9-10,12-18H2,1H3,(H,28,34)(H,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human fibroblast collagenase, matrix metalloproteinase-1 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50284753

((R)-N*1*-[(S)-1-Hexylcarbamoyl-2-(1H-indol-3-yl)-e...)Show SMILES CCCCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C25H38N4O4/c1-4-5-6-9-12-26-25(32)22(14-19-16-27-21-11-8-7-10-20(19)21)28-24(31)18(13-17(2)3)15-23(30)29-33/h7-8,10-11,16-18,22,27,33H,4-6,9,12-15H2,1-3H3,(H,26,32)(H,28,31)(H,29,30)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50531587

(CHEMBL4471322)Show SMILES CC(C)Cc1cc2N([C@@H](CC(N)=O)COc2cc1Cl)C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C23H24ClN3O5/c1-12(2)5-14-7-18-20(9-16(14)24)31-10-15(8-21(25)28)27(18)23(30)13-3-4-19-17(6-13)26-22(29)11-32-19/h3-4,6-7,9,12,15H,5,8,10-11H2,1-2H3,(H2,25,28)(H,26,29)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay |
J Med Chem 62: 1385-1406 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01523
BindingDB Entry DOI: 10.7270/Q2GT5RP6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50077906
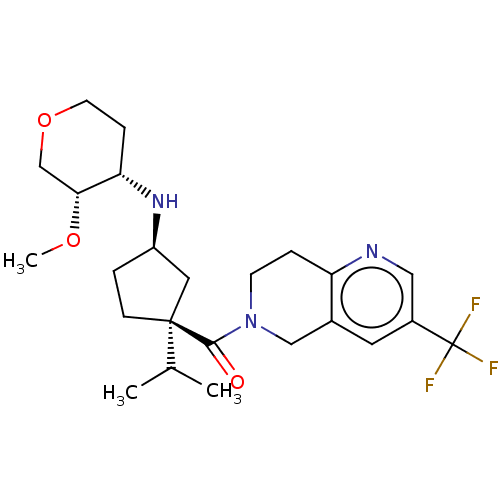
(CHEMBL3305901)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCc2ncc(cc2C1)C(F)(F)F Show InChI InChI=1S/C24H34F3N3O3/c1-15(2)23(7-4-18(11-23)29-20-6-9-33-14-21(20)32-3)22(31)30-8-5-19-16(13-30)10-17(12-28-19)24(25,26)27/h10,12,15,18,20-21,29H,4-9,11,13-14H2,1-3H3/t18-,20+,21-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry |
Eur J Med Chem 93: 121-34 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.063
BindingDB Entry DOI: 10.7270/Q25M67DX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410607
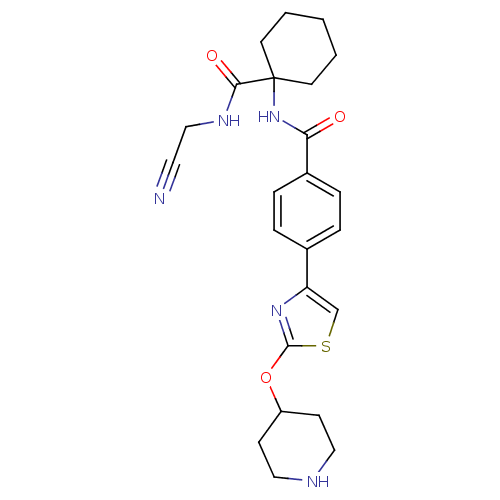
(CHEMBL200744)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(OC2CCNCC2)n1 Show InChI InChI=1S/C24H29N5O3S/c25-12-15-27-22(31)24(10-2-1-3-11-24)29-21(30)18-6-4-17(5-7-18)20-16-33-23(28-20)32-19-8-13-26-14-9-19/h4-7,16,19,26H,1-3,8-11,13-15H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410571
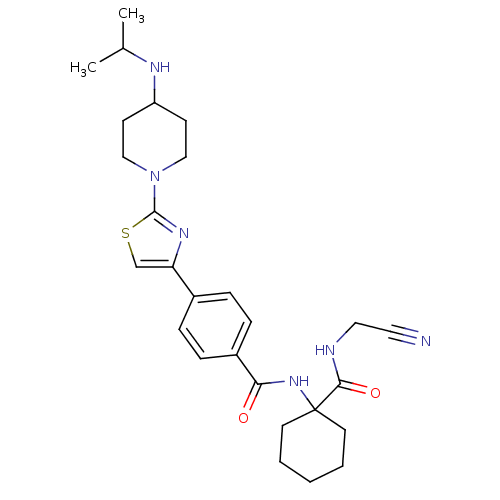
(CHEMBL200287)Show SMILES CC(C)NC1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-19(2)30-22-10-16-33(17-11-22)26-31-23(18-36-26)20-6-8-21(9-7-20)24(34)32-27(12-4-3-5-13-27)25(35)29-15-14-28/h6-9,18-19,22,30H,3-5,10-13,15-17H2,1-2H3,(H,29,35)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50531593
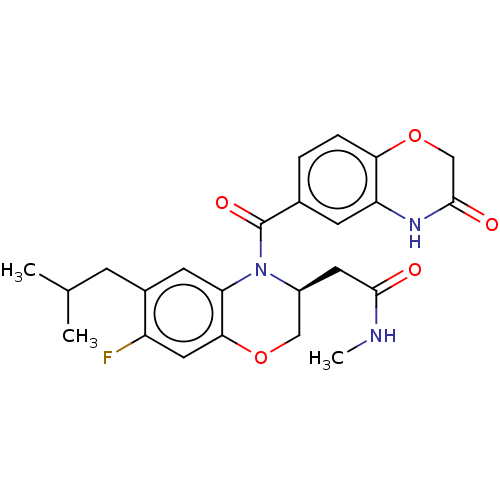
(CHEMBL4555842)Show SMILES CNC(=O)C[C@H]1COc2cc(F)c(CC(C)C)cc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C24H26FN3O5/c1-13(2)6-15-8-19-21(10-17(15)25)32-11-16(9-22(29)26-3)28(19)24(31)14-4-5-20-18(7-14)27-23(30)12-33-20/h4-5,7-8,10,13,16H,6,9,11-12H2,1-3H3,(H,26,29)(H,27,30)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay |
J Med Chem 62: 1385-1406 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01523
BindingDB Entry DOI: 10.7270/Q2GT5RP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410580
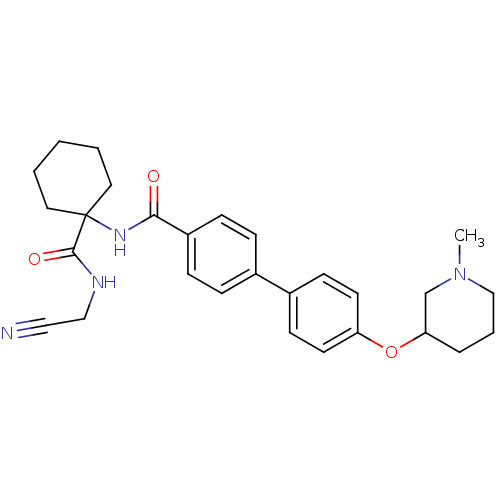
(CHEMBL435913)Show SMILES CN1CCCC(C1)Oc1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H34N4O3/c1-32-19-5-6-25(20-32)35-24-13-11-22(12-14-24)21-7-9-23(10-8-21)26(33)31-28(15-3-2-4-16-28)27(34)30-18-17-29/h7-14,25H,2-6,15-16,18-20H2,1H3,(H,30,34)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410575
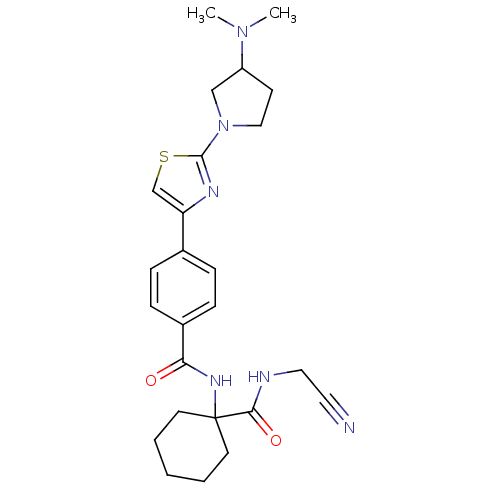
(CHEMBL199470)Show SMILES CN(C)C1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H32N6O2S/c1-30(2)20-10-15-31(16-20)24-28-21(17-34-24)18-6-8-19(9-7-18)22(32)29-25(11-4-3-5-12-25)23(33)27-14-13-26/h6-9,17,20H,3-5,10-12,14-16H2,1-2H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50531569
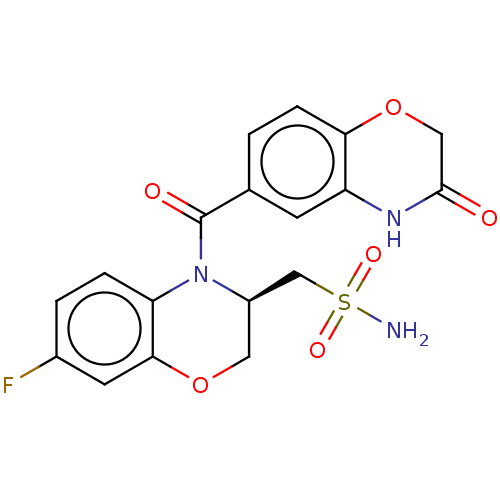
(CHEMBL4532472)Show SMILES NS(=O)(=O)C[C@H]1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C18H16FN3O6S/c19-11-2-3-14-16(6-11)27-7-12(9-29(20,25)26)22(14)18(24)10-1-4-15-13(5-10)21-17(23)8-28-15/h1-6,12H,7-9H2,(H,21,23)(H2,20,25,26)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay |
J Med Chem 62: 1385-1406 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01523
BindingDB Entry DOI: 10.7270/Q2GT5RP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410591

(CHEMBL200506)Show SMILES CN1CCN(Cc2nc(cs2)-c2ccc(cc2)C(=O)NC2(CCCCC2)C(=O)NCC#N)CC1 Show InChI InChI=1S/C25H32N6O2S/c1-30-13-15-31(16-14-30)17-22-28-21(18-34-22)19-5-7-20(8-6-19)23(32)29-25(9-3-2-4-10-25)24(33)27-12-11-26/h5-8,18H,2-4,9-10,12-17H2,1H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50374399

(CHEMBL209293)Show SMILES CCC[C@H](NC(=O)C1(CCCCC1)NC(=O)c1ccc(cc1)N(C)C)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C27H33N5O5/c1-4-9-20(22(33)25-31-30-24(37-25)21-10-8-17-36-21)28-26(35)27(15-6-5-7-16-27)29-23(34)18-11-13-19(14-12-18)32(2)3/h8,10-14,17,20H,4-7,9,15-16H2,1-3H3,(H,28,35)(H,29,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410612
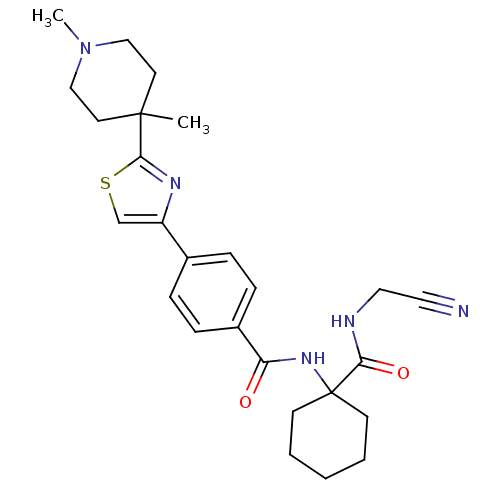
(CHEMBL200166)Show SMILES CN1CCC(C)(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H33N5O2S/c1-25(12-16-31(2)17-13-25)24-29-21(18-34-24)19-6-8-20(9-7-19)22(32)30-26(10-4-3-5-11-26)23(33)28-15-14-27/h6-9,18H,3-5,10-13,15-17H2,1-2H3,(H,28,33)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50284755
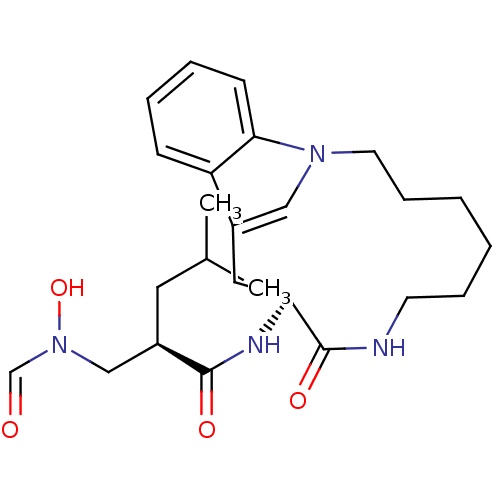
(2-[(Formyl-hydroxy-amino)-methyl]-4-methyl-pentano...)Show SMILES CC(C)C[C@H](CN(O)C=O)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C25H36N4O4/c1-18(2)13-20(16-29(33)17-30)24(31)27-22-14-19-15-28(23-10-6-5-9-21(19)23)12-8-4-3-7-11-26-25(22)32/h5-6,9-10,15,17-18,20,22,33H,3-4,7-8,11-14,16H2,1-2H3,(H,26,32)(H,27,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human fibroblast collagenase, matrix metalloproteinase-1 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50410979

(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM333146
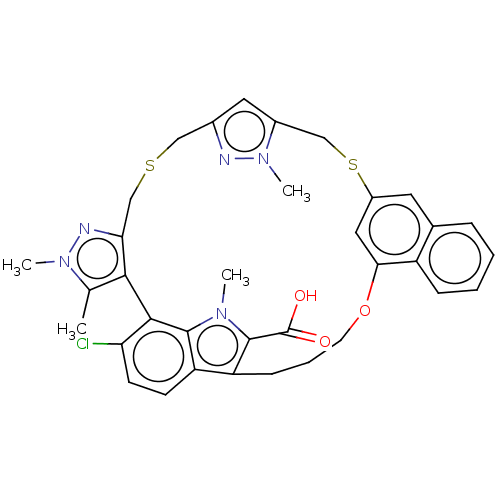
(Compound I | US10196404, Example 1 | US10196404, E...)Show SMILES Cc1c-2c(CSCc3cc(CSc4cc(OCCCc5c(C(O)=O)n(C)c6c-2c(Cl)ccc56)c2ccccc2c4)n(C)n3)nn1C Show InChI InChI=1S/C35H34ClN5O3S2/c1-20-31-29(38-40(20)3)19-45-17-22-15-23(41(4)37-22)18-46-24-14-21-8-5-6-9-25(21)30(16-24)44-13-7-10-26-27-11-12-28(36)32(31)33(27)39(2)34(26)35(42)43/h5-6,8-9,11-12,14-16H,7,10,13,17-19H2,1-4H3,(H,42,43) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-labeled Bak BH3 peptide binding to GST-tagged MCL1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00682
BindingDB Entry DOI: 10.7270/Q2MC93VV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50077924

(CHEMBL3417124)Show SMILES COc1cc2CCC(N[C@@H]3CC[C@](C3)(C(C)C)C(=O)N3CCc4ccc(cc4C3)C(F)(F)F)c2cc1OC |r| Show InChI InChI=1S/C30H37F3N2O3/c1-18(2)29(28(36)35-12-10-19-5-7-22(30(31,32)33)13-21(19)17-35)11-9-23(16-29)34-25-8-6-20-14-26(37-3)27(38-4)15-24(20)25/h5,7,13-15,18,23,25,34H,6,8-12,16-17H2,1-4H3/t23-,25?,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry |
Eur J Med Chem 93: 121-34 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.063
BindingDB Entry DOI: 10.7270/Q25M67DX |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50607111
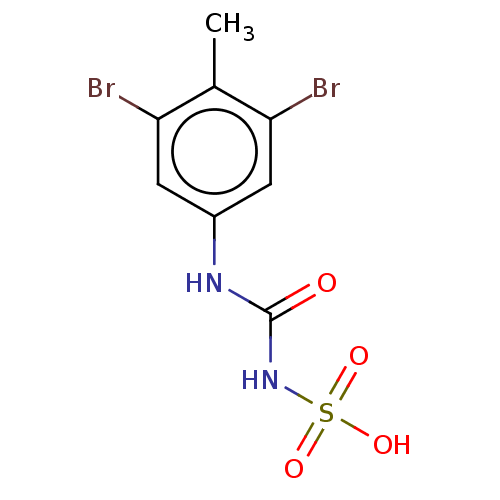
(CHEMBL5218807) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01143
BindingDB Entry DOI: 10.7270/Q2BK1HGF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50519614

(CHEMBL4439276)Show SMILES CN1CCN(CCOc2ccc(-c3c(sc4ncnc(O[C@H](Cc5ccccc5OCc5ccnn5CC(F)(F)F)C(O)=O)c34)-c3ccc(F)o3)c(C)c2Cl)CC1 |r,wD:21.21,(19.44,-4.89,;18.41,-6.03,;18.89,-7.5,;17.87,-8.64,;16.35,-8.32,;15.33,-9.47,;15.81,-10.94,;14.78,-12.09,;15.27,-13.55,;16.78,-13.86,;17.26,-15.32,;16.23,-16.47,;16.71,-17.93,;15.81,-19.18,;16.72,-20.42,;18.18,-19.94,;19.51,-20.71,;20.85,-19.94,;20.85,-18.39,;19.51,-17.62,;19.51,-16.08,;20.84,-15.31,;20.83,-13.77,;22.16,-13,;23.49,-13.77,;24.82,-12.99,;24.82,-11.45,;23.47,-10.69,;22.15,-11.46,;20.81,-10.71,;20.79,-9.17,;22.12,-8.38,;23.53,-9,;24.55,-7.84,;23.76,-6.51,;22.26,-6.85,;21.1,-5.83,;21.09,-4.29,;22.42,-3.51,;19.76,-3.52,;21.09,-2.75,;22.17,-16.08,;23.5,-15.3,;22.18,-17.62,;18.17,-18.4,;14.27,-19.19,;13.36,-17.95,;11.89,-18.43,;11.9,-19.97,;10.66,-20.88,;13.37,-20.44,;14.73,-16.16,;13.7,-17.31,;14.24,-14.71,;12.73,-14.4,;15.87,-6.87,;16.9,-5.72,)| Show InChI InChI=1S/C39H37ClF4N6O6S/c1-23-26(7-8-28(34(23)40)53-18-17-49-15-13-48(2)14-16-49)32-33-36(45-22-46-37(33)57-35(32)29-9-10-31(41)55-29)56-30(38(51)52)19-24-5-3-4-6-27(24)54-20-25-11-12-47-50(25)21-39(42,43)44/h3-12,22,30H,13-21H2,1-2H3,(H,51,52)/t30-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to C-terminal MBP-fused human MCL1 (173 to 321 residues) expressed in Escherichia coli BL21(DE3)pLysS incubated for 2 hrs by fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00682
BindingDB Entry DOI: 10.7270/Q2MC93VV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50531587

(CHEMBL4471322)Show SMILES CC(C)Cc1cc2N([C@@H](CC(N)=O)COc2cc1Cl)C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C23H24ClN3O5/c1-12(2)5-14-7-18-20(9-16(14)24)31-10-15(8-21(25)28)27(18)23(30)13-3-4-19-17(6-13)26-22(29)11-32-19/h3-4,6-7,9,12,15H,5,8,10-11H2,1-2H3,(H2,25,28)(H,26,29)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GR LBD by fluormone GS red-fluorescence polarization assay |
J Med Chem 62: 1385-1406 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01523
BindingDB Entry DOI: 10.7270/Q2GT5RP6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410595
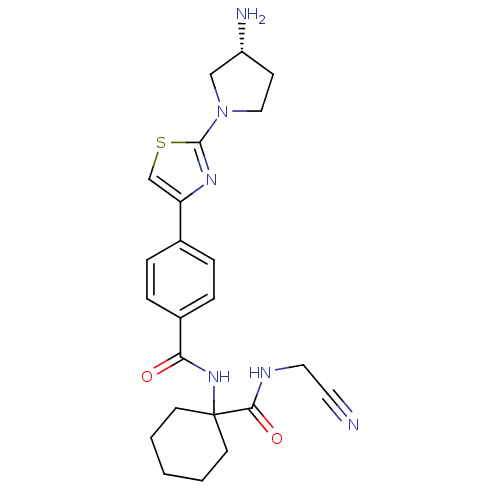
(CHEMBL200507)Show SMILES N[C@@H]1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H28N6O2S/c24-11-12-26-21(31)23(9-2-1-3-10-23)28-20(30)17-6-4-16(5-7-17)19-15-32-22(27-19)29-13-8-18(25)14-29/h4-7,15,18H,1-3,8-10,12-14,25H2,(H,26,31)(H,28,30)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19855
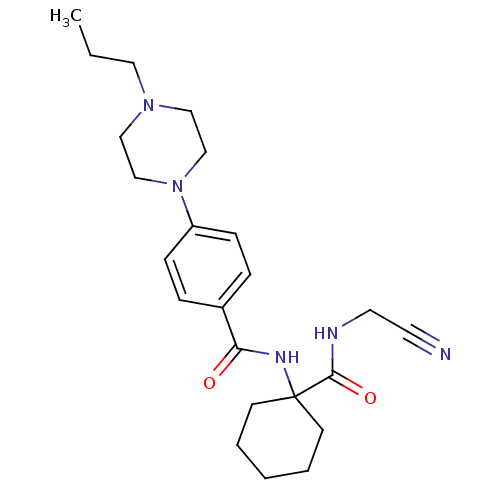
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410572
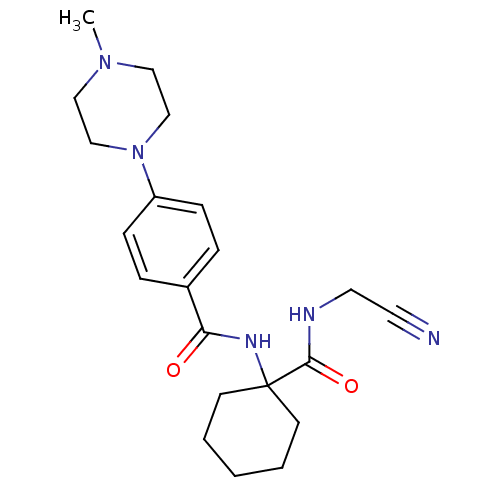
(CHEMBL440035)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C21H29N5O2/c1-25-13-15-26(16-14-25)18-7-5-17(6-8-18)19(27)24-21(9-3-2-4-10-21)20(28)23-12-11-22/h5-8H,2-4,9-10,12-16H2,1H3,(H,23,28)(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410592
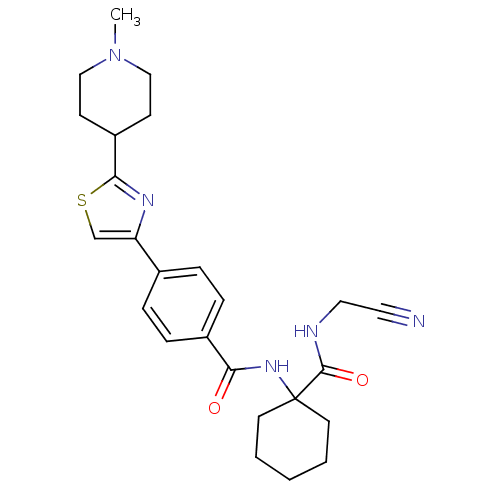
(CHEMBL200455)Show SMILES CN1CCC(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H31N5O2S/c1-30-15-9-20(10-16-30)23-28-21(17-33-23)18-5-7-19(8-6-18)22(31)29-25(11-3-2-4-12-25)24(32)27-14-13-26/h5-8,17,20H,2-4,9-12,14-16H2,1H3,(H,27,32)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410594
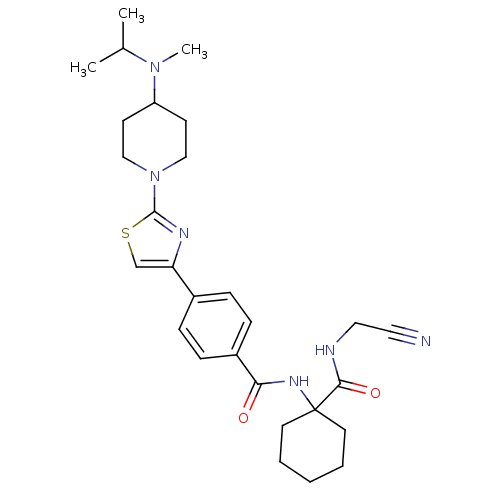
(CHEMBL200596)Show SMILES CC(C)N(C)C1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H38N6O2S/c1-20(2)33(3)23-11-17-34(18-12-23)27-31-24(19-37-27)21-7-9-22(10-8-21)25(35)32-28(13-5-4-6-14-28)26(36)30-16-15-29/h7-10,19-20,23H,4-6,11-14,16-18H2,1-3H3,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50077980
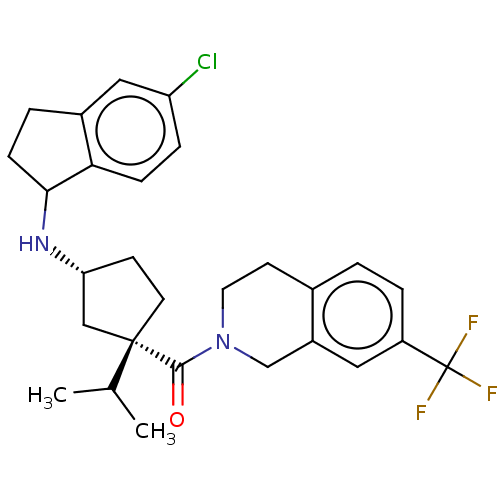
(CHEMBL3417117)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCc2cc(Cl)ccc12)C(=O)N1CCc2ccc(cc2C1)C(F)(F)F |r| Show InChI InChI=1S/C28H32ClF3N2O/c1-17(2)27(11-9-23(15-27)33-25-8-4-19-14-22(29)6-7-24(19)25)26(35)34-12-10-18-3-5-21(28(30,31)32)13-20(18)16-34/h3,5-7,13-14,17,23,25,33H,4,8-12,15-16H2,1-2H3/t23-,25?,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry |
Eur J Med Chem 93: 121-34 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.063
BindingDB Entry DOI: 10.7270/Q25M67DX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50095652
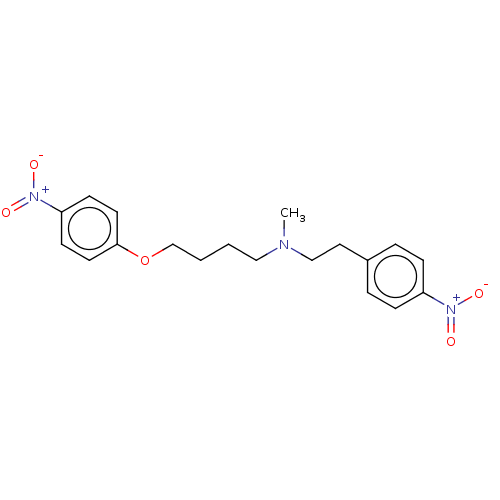
(CHEMBL3590452)Show SMILES CN(CCCCOc1ccc(cc1)[N+]([O-])=O)CCc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H23N3O5/c1-20(14-12-16-4-6-17(7-5-16)21(23)24)13-2-3-15-27-19-10-8-18(9-11-19)22(25)26/h4-11H,2-3,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human Kv11.1 expressed in HEK293 cell membranes by competitive radioligand displacement assay |
J Med Chem 58: 5916-29 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00518
BindingDB Entry DOI: 10.7270/Q2FX7C6V |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50531563

(CHEMBL4463240)Show SMILES CC(C)Cc1cc2N([C@@H](CS(N)(=O)=O)COc2cc1F)C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C22H24FN3O6S/c1-12(2)5-14-7-18-20(8-16(14)23)31-9-15(11-33(24,29)30)26(18)22(28)13-3-4-19-17(6-13)25-21(27)10-32-19/h3-4,6-8,12,15H,5,9-11H2,1-2H3,(H,25,27)(H2,24,29,30)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GR LBD by fluormone GS red-fluorescence polarization assay |
J Med Chem 62: 1385-1406 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01523
BindingDB Entry DOI: 10.7270/Q2GT5RP6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50374365

(CHEMBL209215)Show SMILES CCC[C@H](NC(=O)C1(CCCCC1)NC(=O)c1ccc(OC(F)(F)F)cc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C26H27F3N4O6/c1-2-7-18(20(34)23-33-32-22(38-23)19-8-6-15-37-19)30-24(36)25(13-4-3-5-14-25)31-21(35)16-9-11-17(12-10-16)39-26(27,28)29/h6,8-12,15,18H,2-5,7,13-14H2,1H3,(H,30,36)(H,31,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50095764
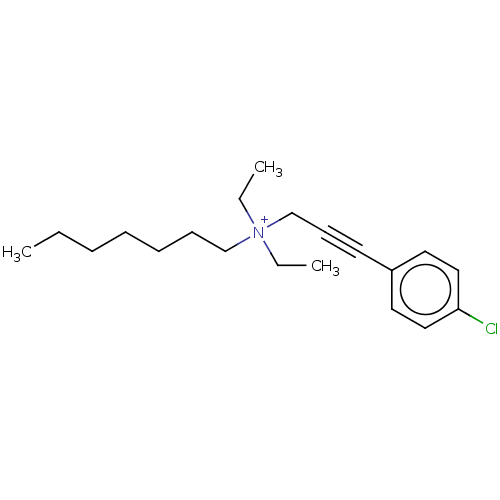
(CHEMBL3140084)Show InChI InChI=1S/C14H15N3O/c1-10-4-3-5-11(2)13(10)17-14(18)16-12-6-8-15-9-7-12/h3-9H,1-2H3,(H2,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human Kv11.1 expressed in HEK293 cell membranes by competitive radioligand displacement assay |
J Med Chem 58: 5916-29 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00518
BindingDB Entry DOI: 10.7270/Q2FX7C6V |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50410979

(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data