| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Ubiquitin carboxyl-terminal hydrolase isozyme L1 |
|---|
| Ligand | BDBM445166 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | UCHL1 Biochemical IC50 Assay |
|---|
| IC50 | 550±n/a nM |
|---|
| Citation |  Kemp, M; Stockley, M; Jones, A Cyanopyrrolidines as dub inhibitors for the treatment of cancer US Patent US10669234 Publication Date 6/2/2020 Kemp, M; Stockley, M; Jones, A Cyanopyrrolidines as dub inhibitors for the treatment of cancer US Patent US10669234 Publication Date 6/2/2020 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Ubiquitin carboxyl-terminal hydrolase isozyme L1 |
|---|
| Name: | Ubiquitin carboxyl-terminal hydrolase isozyme L1 |
|---|
| Synonyms: | Neuron cytoplasmic protein 9.5 | PGP 9.5 | PGP9.5 | UCH-L1 | UCHL1 | UCHL1_HUMAN | Ubiquitin thioesterase L1 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 24819.03 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_974327 |
|---|
| Residue: | 223 |
|---|
| Sequence: | MQLKPMEINPEMLNKVLSRLGVAGQWRFVDVLGLEEESLGSVPAPACALLLLFPLTAQHE
NFRKKQIEELKGQEVSPKVYFMKQTIGNSCGTIGLIHAVANNQDKLGFEDGSVLKQFLSE
TEKMSPEDRAKCFEKNEAIQAAHDAVAQEGQCRVDDKVNFHFILFNNVDGHLYELDGRMP
FPVNHGASSEDTLLKDAAKVCREFTEREQGEVRFSAVALCKAA
|
|
|
|---|
| BDBM445166 |
|---|
| n/a |
|---|
| Name | BDBM445166 |
|---|
| Synonyms: | (S)-2-(4-(5H-pyrrolo[2,3- b]pyrazin-7-yl)indoline-1- carbonyl)pyrrolidine-1- carbonitrile | US10669234, Example 123 | US11319287, Example 123 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H18N6O |
|---|
| Mol. Mass. | 358.3965 |
|---|
| SMILES | O=C([C@@H]1CCCN1C#N)N1CCc2c1cccc2-c1c[nH]c2nccnc12 |
|---|
| Structure | 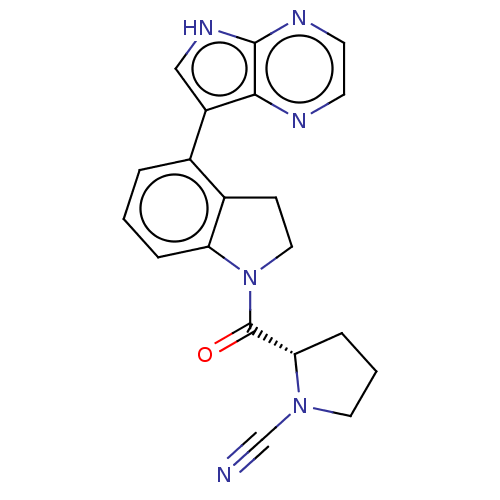
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kemp, M; Stockley, M; Jones, A Cyanopyrrolidines as dub inhibitors for the treatment of cancer US Patent US10669234 Publication Date 6/2/2020
Kemp, M; Stockley, M; Jones, A Cyanopyrrolidines as dub inhibitors for the treatment of cancer US Patent US10669234 Publication Date 6/2/2020