| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50249046 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1679702 (CHEMBL4029979) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Hoyt, SB; Taylor, J; London, C; Ali, A; Ujjainwalla, F; Tata, J; Struthers, M; Cully, D; Wisniewski, T; Ren, N; Bopp, C; Sok, A; Verras, A; McMasters, D; Chen, Q; Tung, E; Tang, W; Salituro, G; Clemas, J; Zhou, G; MacNeil, D; Duffy, R; Xiong, Y Discovery of indazole aldosterone synthase (CYP11B2) inhibitors as potential treatments for hypertension. Bioorg Med Chem Lett27:2384-2388 (2017) [PubMed] Article Hoyt, SB; Taylor, J; London, C; Ali, A; Ujjainwalla, F; Tata, J; Struthers, M; Cully, D; Wisniewski, T; Ren, N; Bopp, C; Sok, A; Verras, A; McMasters, D; Chen, Q; Tung, E; Tang, W; Salituro, G; Clemas, J; Zhou, G; MacNeil, D; Duffy, R; Xiong, Y Discovery of indazole aldosterone synthase (CYP11B2) inhibitors as potential treatments for hypertension. Bioorg Med Chem Lett27:2384-2388 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50249046 |
|---|
| n/a |
|---|
| Name | BDBM50249046 |
|---|
| Synonyms: | CHEMBL4071689 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H11F6N3O |
|---|
| Mol. Mass. | 375.2685 |
|---|
| SMILES | C[C@](O)(c1cncc(c1)-n1nc(c2ccccc12)C(F)(F)F)C(F)(F)F |r| |
|---|
| Structure | 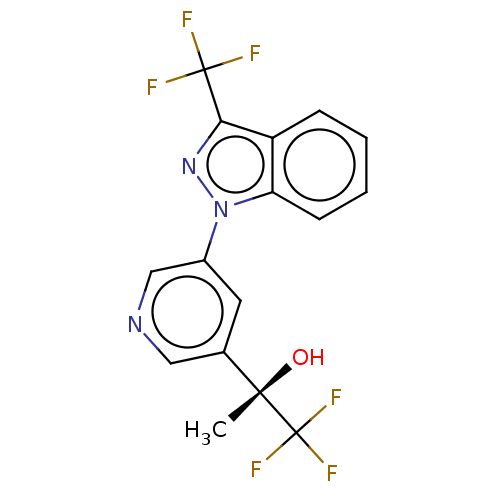
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hoyt, SB; Taylor, J; London, C; Ali, A; Ujjainwalla, F; Tata, J; Struthers, M; Cully, D; Wisniewski, T; Ren, N; Bopp, C; Sok, A; Verras, A; McMasters, D; Chen, Q; Tung, E; Tang, W; Salituro, G; Clemas, J; Zhou, G; MacNeil, D; Duffy, R; Xiong, Y Discovery of indazole aldosterone synthase (CYP11B2) inhibitors as potential treatments for hypertension. Bioorg Med Chem Lett27:2384-2388 (2017) [PubMed] Article
Hoyt, SB; Taylor, J; London, C; Ali, A; Ujjainwalla, F; Tata, J; Struthers, M; Cully, D; Wisniewski, T; Ren, N; Bopp, C; Sok, A; Verras, A; McMasters, D; Chen, Q; Tung, E; Tang, W; Salituro, G; Clemas, J; Zhou, G; MacNeil, D; Duffy, R; Xiong, Y Discovery of indazole aldosterone synthase (CYP11B2) inhibitors as potential treatments for hypertension. Bioorg Med Chem Lett27:2384-2388 (2017) [PubMed] Article