 Found 568 hits with Last Name = 'tung' and Initial = 'e'
Found 568 hits with Last Name = 'tung' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443348
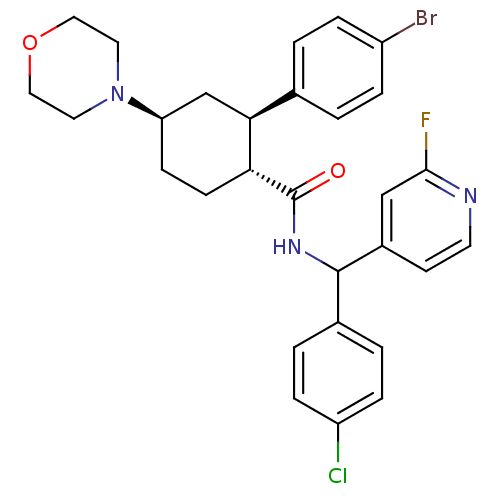
(CHEMBL3086040 | US8669252, 12)Show SMILES Fc1cc(ccn1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443351
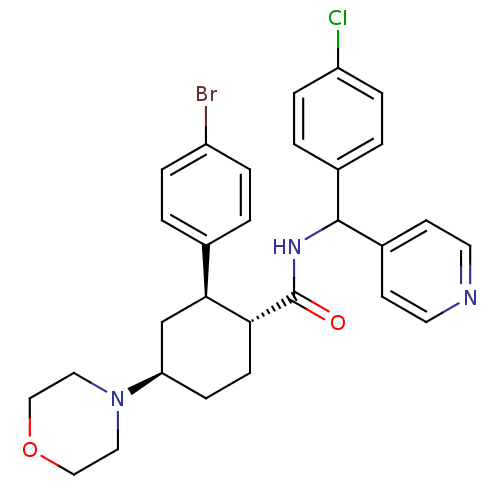
(CHEMBL3086037 | US8669252, 15)Show SMILES Clc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccncc1 |r| Show InChI InChI=1S/C29H31BrClN3O2/c30-23-5-1-20(2-6-23)27-19-25(34-15-17-36-18-16-34)9-10-26(27)29(35)33-28(22-11-13-32-14-12-22)21-3-7-24(31)8-4-21/h1-8,11-14,25-28H,9-10,15-19H2,(H,33,35)/t25-,26-,27+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383421
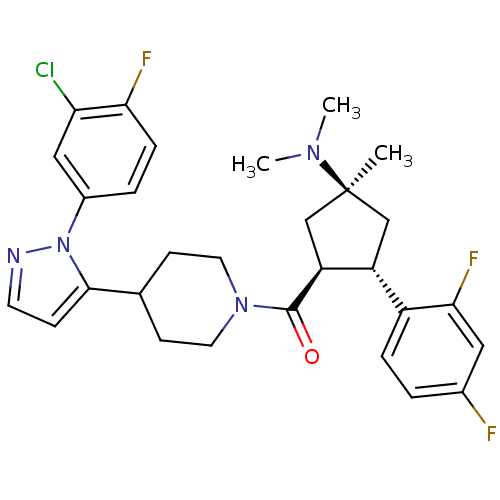
(CHEMBL2031595)Show SMILES CN(C)[C@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382913
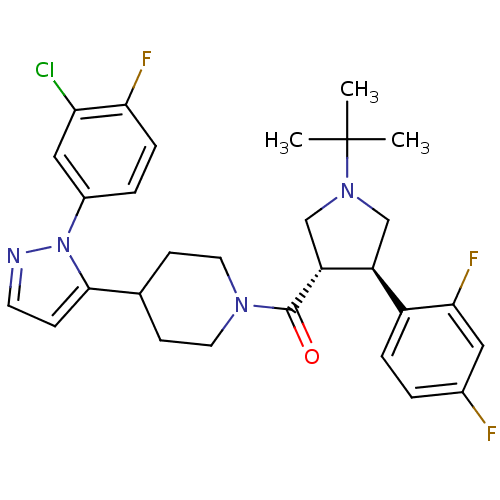
(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092190
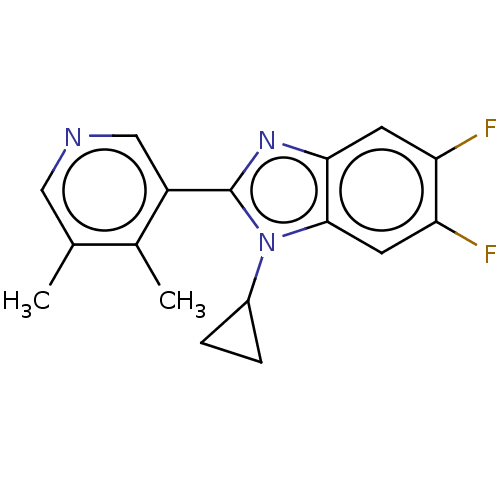
(CHEMBL3582482)Show InChI InChI=1S/C17H15F2N3/c1-9-7-20-8-12(10(9)2)17-21-15-5-13(18)14(19)6-16(15)22(17)11-3-4-11/h5-8,11H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382904
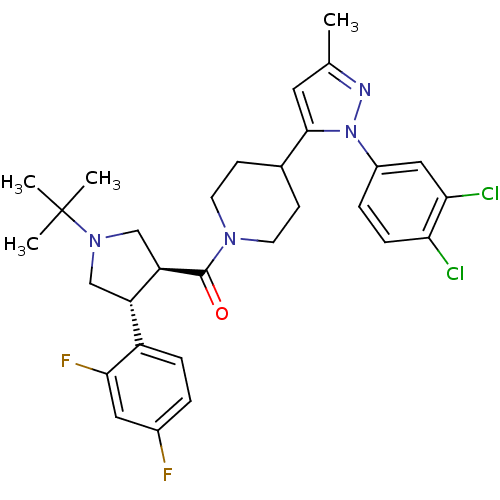
(CHEMBL2022793)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)21-6-8-25(31)26(32)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(33)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382899

(CHEMBL2022787)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(C)c(Cl)c1 |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-6-8-23(16-27(19)32)38-29(14-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-9-7-22(33)15-28(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50249076
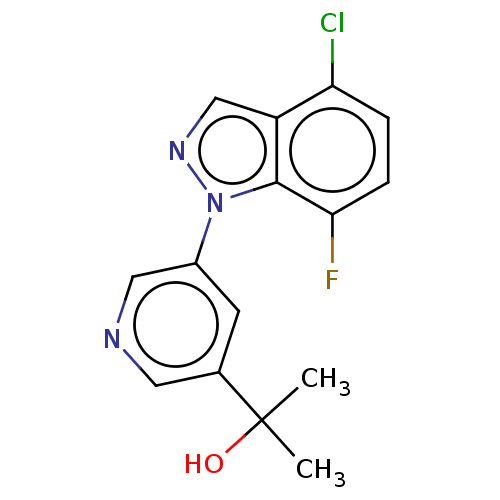
(CHEMBL4096914)Show InChI InChI=1S/C15H13ClFN3O/c1-15(2,21)9-5-10(7-18-6-9)20-14-11(8-19-20)12(16)3-4-13(14)17/h3-8,21H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2-CLE9 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by s... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50443348

(CHEMBL3086040 | US8669252, 12)Show SMILES Fc1cc(ccn1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382913

(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50249075
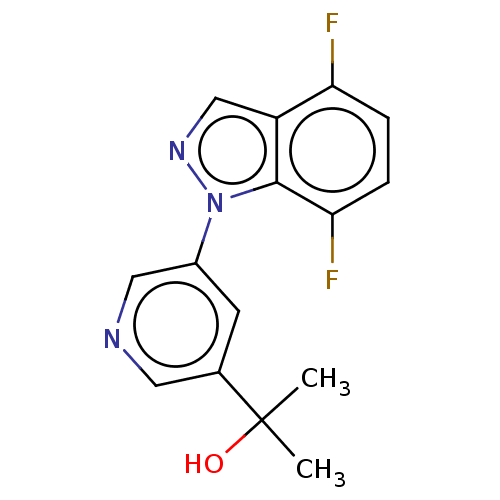
(CHEMBL4069720)Show InChI InChI=1S/C15H13F2N3O/c1-15(2,21)9-5-10(7-18-6-9)20-14-11(8-19-20)12(16)3-4-13(14)17/h3-8,21H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2-CLE9 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by s... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092142

(CHEMBL3582477)Show SMILES CC(C)(O)c1cncc(c1)-c1nc2cc(F)c(F)cc2n1C1CC1 Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6-,8+,9-,10+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361786
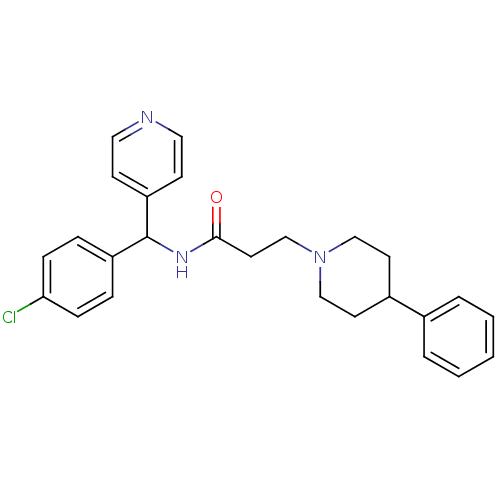
(CHEMBL1938522)Show SMILES Clc1ccc(cc1)C(NC(=O)CCN1CCC(CC1)c1ccccc1)c1ccncc1 Show InChI InChI=1S/C26H28ClN3O/c27-24-8-6-22(7-9-24)26(23-10-15-28-16-11-23)29-25(31)14-19-30-17-12-21(13-18-30)20-4-2-1-3-5-20/h1-11,15-16,21,26H,12-14,17-19H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382905

(CHEMBL2022794)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)27-8-5-20(31)14-25(27)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383421

(CHEMBL2031595)Show SMILES CN(C)[C@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50361774
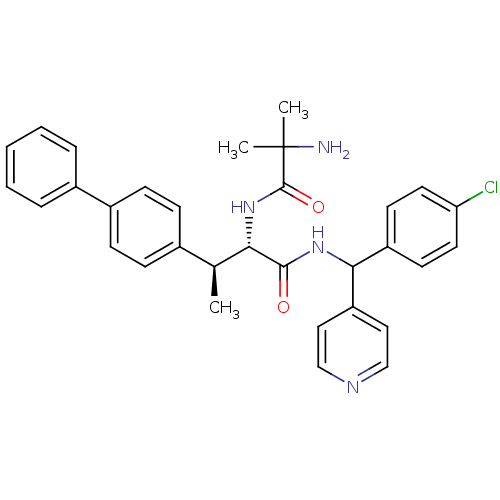
(CHEMBL1938510)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)NC(c1ccncc1)c1ccc(Cl)cc1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C32H33ClN4O2/c1-21(22-9-11-24(12-10-22)23-7-5-4-6-8-23)28(37-31(39)32(2,3)34)30(38)36-29(26-17-19-35-20-18-26)25-13-15-27(33)16-14-25/h4-21,28-29H,34H2,1-3H3,(H,36,38)(H,37,39)/t21-,28-,29?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092182
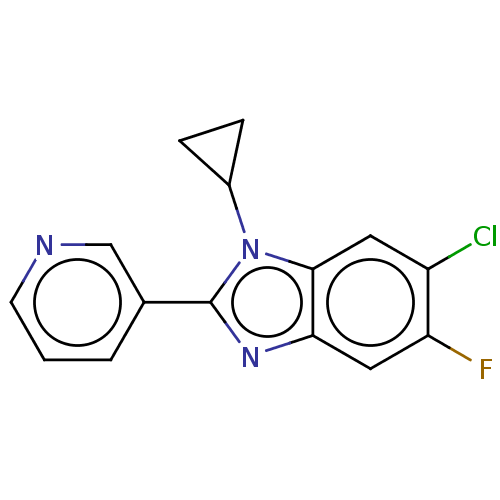
(CHEMBL3582470)Show InChI InChI=1S/C15H11ClFN3/c16-11-6-14-13(7-12(11)17)19-15(20(14)10-3-4-10)9-2-1-5-18-8-9/h1-2,5-8,10H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092185
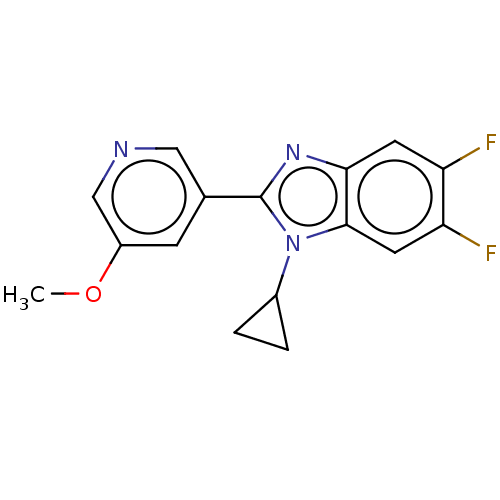
(CHEMBL3582474)Show InChI InChI=1S/C16H13F2N3O/c1-22-11-4-9(7-19-8-11)16-20-14-5-12(17)13(18)6-15(14)21(16)10-2-3-10/h4-8,10H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383427
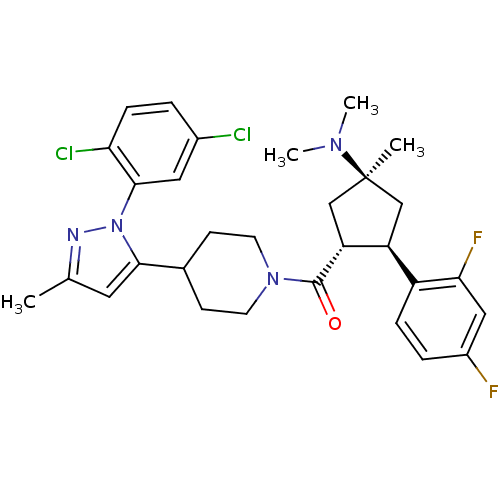
(CHEMBL2031588)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092137
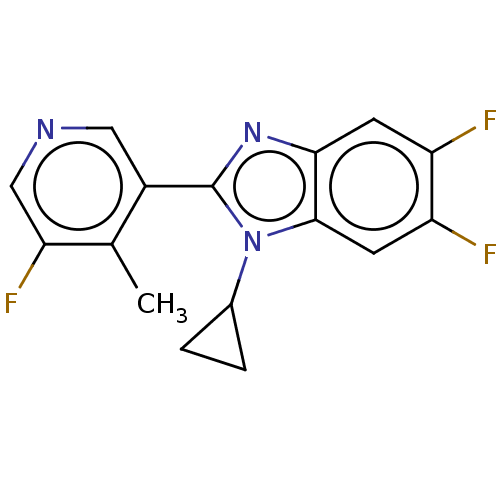
(CHEMBL3582481)Show InChI InChI=1S/C16H12F3N3/c1-8-10(6-20-7-13(8)19)16-21-14-4-11(17)12(18)5-15(14)22(16)9-2-3-9/h4-7,9H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383425
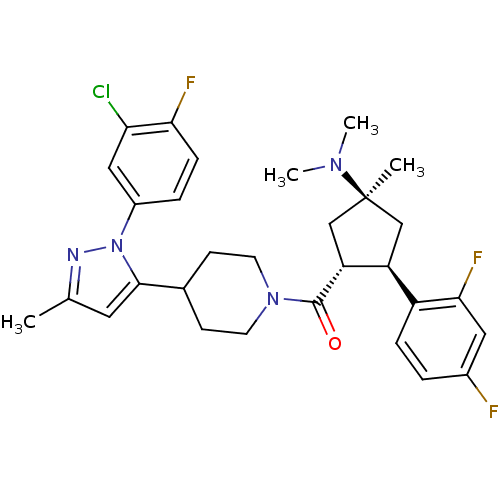
(CHEMBL2031590)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382912

(CHEMBL2023209 | US8569299, 4)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382910
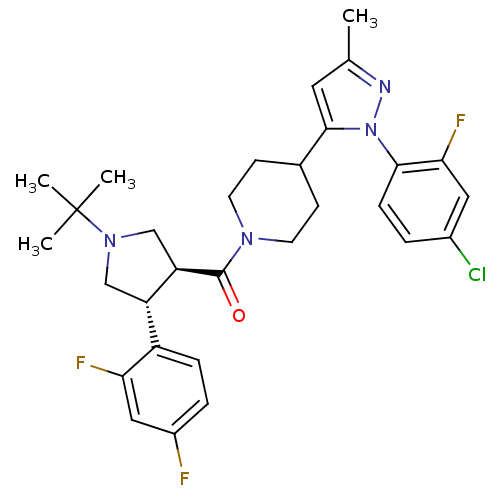
(CHEMBL2023207)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1F |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)27-8-5-20(31)14-26(27)34)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(32)15-25(22)33/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361774

(CHEMBL1938510)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)NC(c1ccncc1)c1ccc(Cl)cc1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C32H33ClN4O2/c1-21(22-9-11-24(12-10-22)23-7-5-4-6-8-23)28(37-31(39)32(2,3)34)30(38)36-29(26-17-19-35-20-18-26)25-13-15-27(33)16-14-25/h4-21,28-29H,34H2,1-3H3,(H,36,38)(H,37,39)/t21-,28-,29?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092183
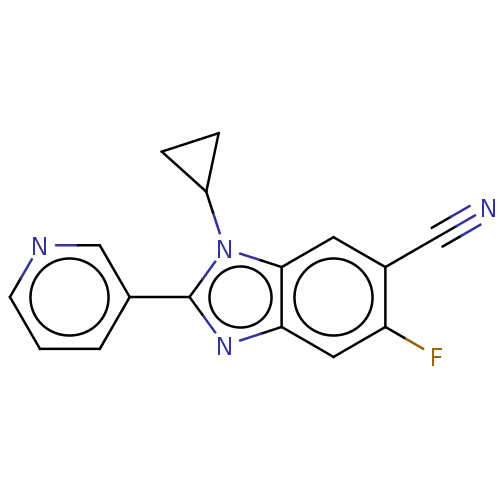
(CHEMBL3582471)Show InChI InChI=1S/C16H11FN4/c17-13-7-14-15(6-11(13)8-18)21(12-3-4-12)16(20-14)10-2-1-5-19-9-10/h1-2,5-7,9,12H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092191
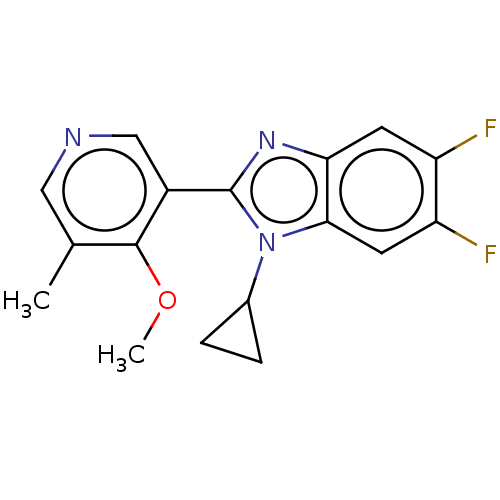
(CHEMBL3582483)Show InChI InChI=1S/C17H15F2N3O/c1-9-7-20-8-11(16(9)23-2)17-21-14-5-12(18)13(19)6-15(14)22(17)10-3-4-10/h5-8,10H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443352
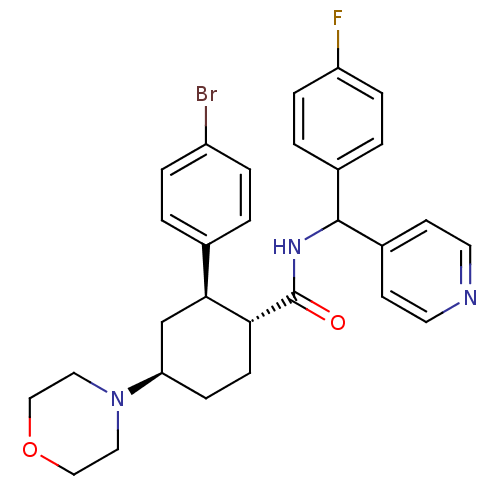
(CHEMBL3085780)Show SMILES Fc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccncc1 |r| Show InChI InChI=1S/C29H31BrFN3O2/c30-23-5-1-20(2-6-23)27-19-25(34-15-17-36-18-16-34)9-10-26(27)29(35)33-28(22-11-13-32-14-12-22)21-3-7-24(31)8-4-21/h1-8,11-14,25-28H,9-10,15-19H2,(H,33,35)/t25-,26-,27+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443353
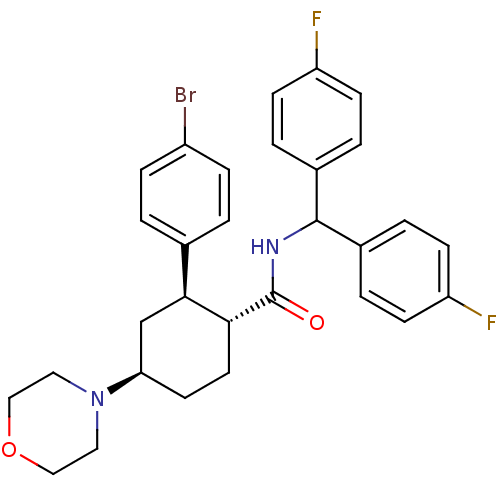
(CHEMBL3086036 | US8669252, 14)Show SMILES Fc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H31BrF2N2O2/c31-23-7-1-20(2-8-23)28-19-26(35-15-17-37-18-16-35)13-14-27(28)30(36)34-29(21-3-9-24(32)10-4-21)22-5-11-25(33)12-6-22/h1-12,26-29H,13-19H2,(H,34,36)/t26-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50001308
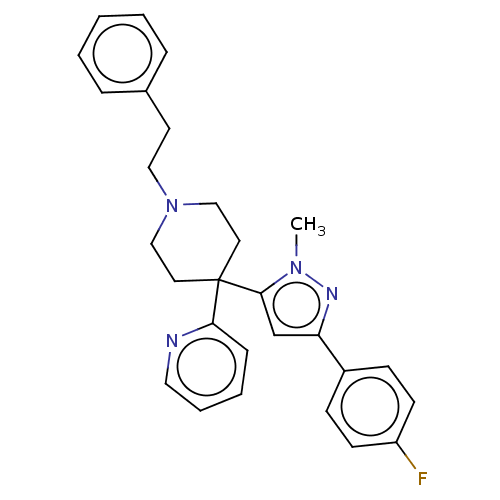
(CHEMBL3237393)Show SMILES OC(=O)C(F)(F)F.Cn1nc(cc1C1(CCN(CCc2ccccc2)CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C28H29FN4.C2HF3O2/c1-32-27(21-25(31-32)23-10-12-24(29)13-11-23)28(26-9-5-6-17-30-26)15-19-33(20-16-28)18-14-22-7-3-2-4-8-22;3-2(4,5)1(6)7/h2-13,17,21H,14-16,18-20H2,1H3;(H,6,7) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate after 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 24: 1657-60 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.070
BindingDB Entry DOI: 10.7270/Q2GB25JD |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382898
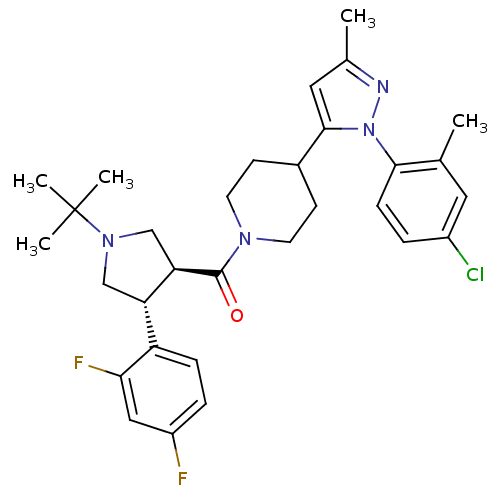
(CHEMBL2022786)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1C |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-14-22(32)6-9-28(19)38-29(15-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-8-7-23(33)16-27(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50443351

(CHEMBL3086037 | US8669252, 15)Show SMILES Clc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccncc1 |r| Show InChI InChI=1S/C29H31BrClN3O2/c30-23-5-1-20(2-6-23)27-19-25(34-15-17-36-18-16-34)9-10-26(27)29(35)33-28(22-11-13-32-14-12-22)21-3-7-24(31)8-4-21/h1-8,11-14,25-28H,9-10,15-19H2,(H,33,35)/t25-,26-,27+,28?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092186
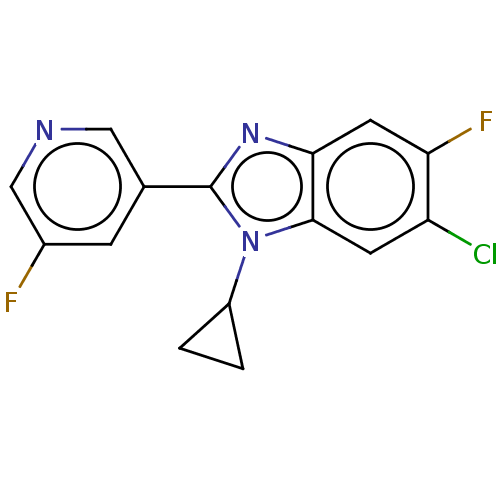
(CHEMBL3582475)Show InChI InChI=1S/C15H10ClF2N3/c16-11-4-14-13(5-12(11)18)20-15(21(14)10-1-2-10)8-3-9(17)7-19-6-8/h3-7,10H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382912

(CHEMBL2023209 | US8569299, 4)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383420
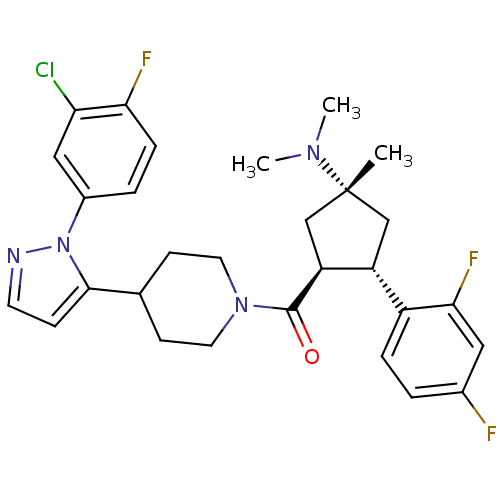
(CHEMBL2031596)Show SMILES CN(C)[C@@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361766

(CHEMBL1938502)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H32ClN3O2/c1-18(20-10-12-23(13-11-20)22-8-6-5-7-9-22)25(32-27(34)28(3,4)30)26(33)31-19(2)21-14-16-24(29)17-15-21/h5-19,25H,30H2,1-4H3,(H,31,33)(H,32,34)/t18-,19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382886
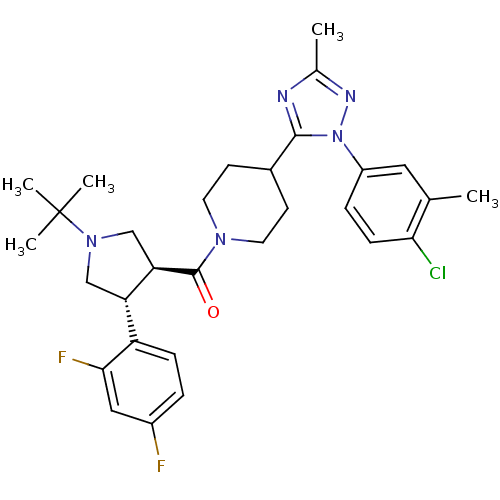
(CHEMBL2024198 | US8569299, 23)Show SMILES Cc1nc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(C)c1 |r| Show InChI InChI=1S/C30H36ClF2N5O/c1-18-14-22(7-9-26(18)31)38-28(34-19(2)35-38)20-10-12-36(13-11-20)29(39)25-17-37(30(3,4)5)16-24(25)23-8-6-21(32)15-27(23)33/h6-9,14-15,20,24-25H,10-13,16-17H2,1-5H3/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382886

(CHEMBL2024198 | US8569299, 23)Show SMILES Cc1nc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(C)c1 |r| Show InChI InChI=1S/C30H36ClF2N5O/c1-18-14-22(7-9-26(18)31)38-28(34-19(2)35-38)20-10-12-36(13-11-20)29(39)25-17-37(30(3,4)5)16-24(25)23-8-6-21(32)15-27(23)33/h6-9,14-15,20,24-25H,10-13,16-17H2,1-5H3/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383425

(CHEMBL2031590)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383426
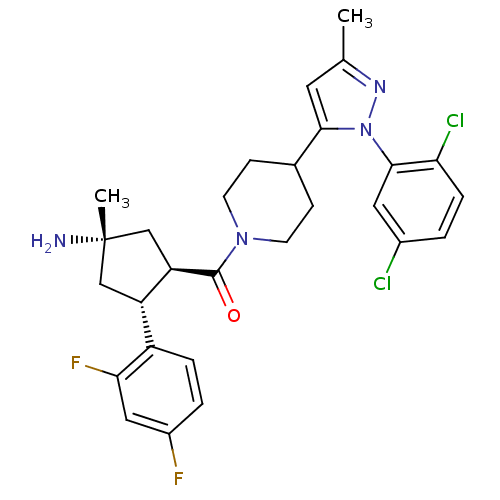
(CHEMBL2031589)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2C[C@@](C)(N)C[C@H]2c2ccc(F)cc2F)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C28H30Cl2F2N4O/c1-16-11-25(36(34-16)26-12-18(29)3-6-23(26)30)17-7-9-35(10-8-17)27(37)22-15-28(2,33)14-21(22)20-5-4-19(31)13-24(20)32/h3-6,11-13,17,21-22H,7-10,14-15,33H2,1-2H3/t21-,22+,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50249064
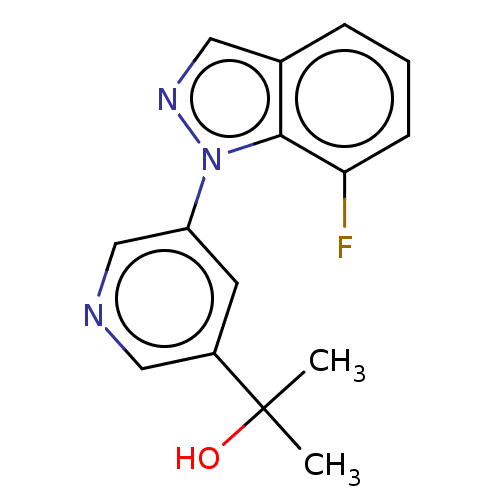
(CHEMBL4075377)Show InChI InChI=1S/C15H14FN3O/c1-15(2,20)11-6-12(9-17-8-11)19-14-10(7-18-19)4-3-5-13(14)16/h3-9,20H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2-CLE9 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by s... |
Bioorg Med Chem Lett 27: 2384-2388 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.021
BindingDB Entry DOI: 10.7270/Q2MC92F2 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383427

(CHEMBL2031588)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443347
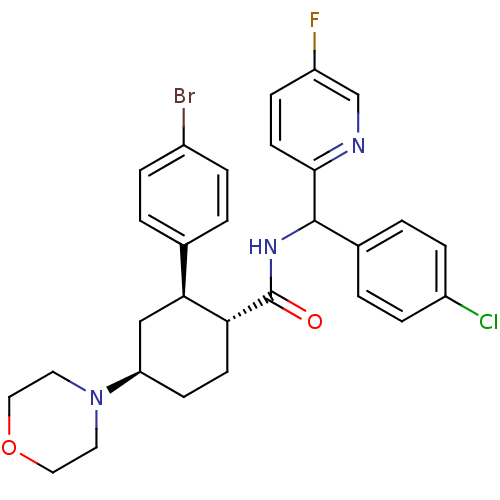
(CHEMBL3086041 | US8669252, 28)Show SMILES Fc1ccc(nc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-21-5-1-19(2-6-21)26-17-24(35-13-15-37-16-14-35)10-11-25(26)29(36)34-28(20-3-7-22(31)8-4-20)27-12-9-23(32)18-33-27/h1-9,12,18,24-26,28H,10-11,13-17H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382904

(CHEMBL2022793)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)21-6-8-25(31)26(32)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(33)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383419
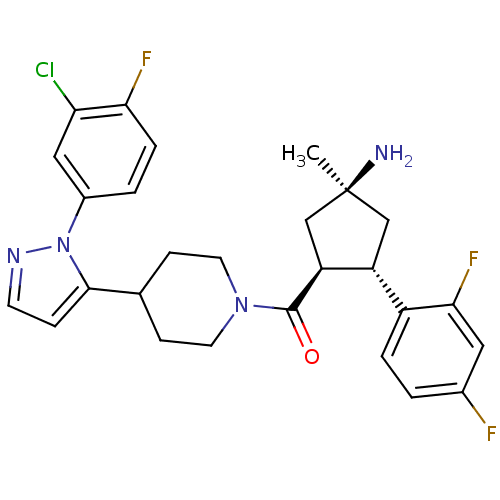
(CHEMBL2031597)Show SMILES C[C@@]1(N)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C27H28ClF3N4O/c1-27(32)14-20(19-4-2-17(29)12-24(19)31)21(15-27)26(36)34-10-7-16(8-11-34)25-6-9-33-35(25)18-3-5-23(30)22(28)13-18/h2-6,9,12-13,16,20-21H,7-8,10-11,14-15,32H2,1H3/t20-,21+,27+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382900
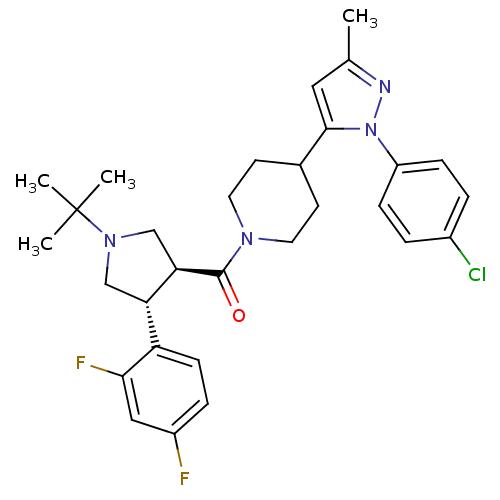
(CHEMBL2022789)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H35ClF2N4O/c1-19-15-28(37(34-19)23-8-5-21(31)6-9-23)20-11-13-35(14-12-20)29(38)26-18-36(30(2,3)4)17-25(26)24-10-7-22(32)16-27(24)33/h5-10,15-16,20,25-26H,11-14,17-18H2,1-4H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523
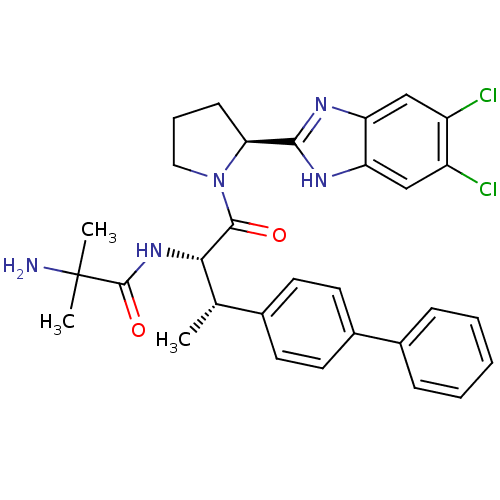
(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data