| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50536173 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1933017 (CHEMBL4478669) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Lapierre, JM; Eathiraj, S; Vensel, D; Liu, Y; Bull, CO; Cornell-Kennon, S; Iimura, S; Kelleher, EW; Kizer, DE; Koerner, S; Makhija, S; Matsuda, A; Moussa, M; Namdev, N; Savage, RE; Szwaya, J; Volckova, E; Westlund, N; Wu, H; Schwartz, B Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J Med Chem59:6455-69 (2016) [PubMed] Article Lapierre, JM; Eathiraj, S; Vensel, D; Liu, Y; Bull, CO; Cornell-Kennon, S; Iimura, S; Kelleher, EW; Kizer, DE; Koerner, S; Makhija, S; Matsuda, A; Moussa, M; Namdev, N; Savage, RE; Szwaya, J; Volckova, E; Westlund, N; Wu, H; Schwartz, B Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J Med Chem59:6455-69 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50536173 |
|---|
| n/a |
|---|
| Name | BDBM50536173 |
|---|
| Synonyms: | CHEMBL4546484 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H24ClN7O |
|---|
| Mol. Mass. | 485.968 |
|---|
| SMILES | Cl.CC(=O)Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(CN)cc3)c2n1 |
|---|
| Structure | 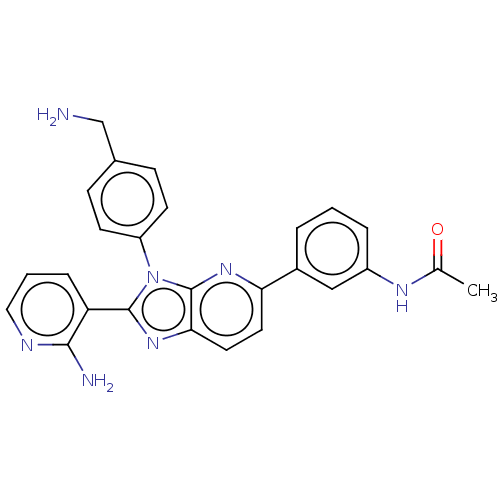
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lapierre, JM; Eathiraj, S; Vensel, D; Liu, Y; Bull, CO; Cornell-Kennon, S; Iimura, S; Kelleher, EW; Kizer, DE; Koerner, S; Makhija, S; Matsuda, A; Moussa, M; Namdev, N; Savage, RE; Szwaya, J; Volckova, E; Westlund, N; Wu, H; Schwartz, B Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J Med Chem59:6455-69 (2016) [PubMed] Article
Lapierre, JM; Eathiraj, S; Vensel, D; Liu, Y; Bull, CO; Cornell-Kennon, S; Iimura, S; Kelleher, EW; Kizer, DE; Koerner, S; Makhija, S; Matsuda, A; Moussa, M; Namdev, N; Savage, RE; Szwaya, J; Volckova, E; Westlund, N; Wu, H; Schwartz, B Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J Med Chem59:6455-69 (2016) [PubMed] Article