

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92766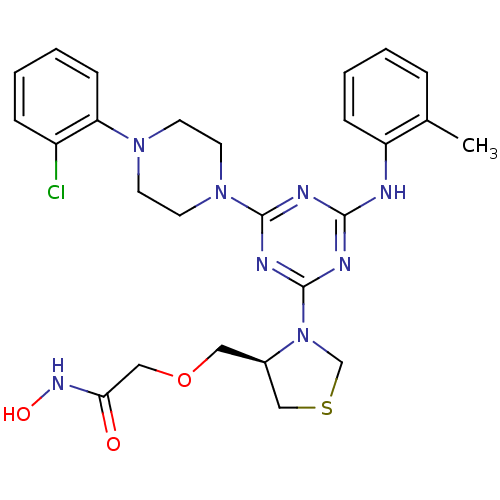 (PDF inhibitor, compound 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92761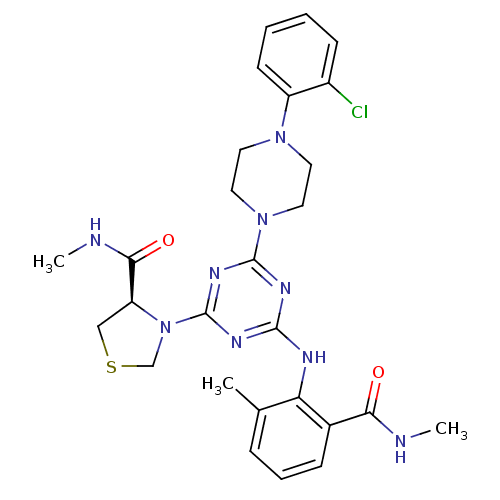 (PDF inhibitor, compound 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92764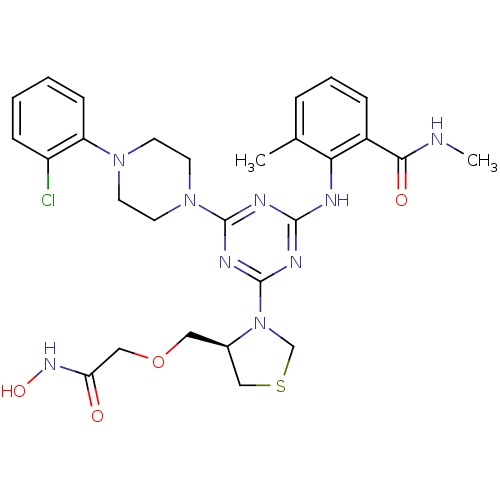 (PDF inhibitor, compound 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92760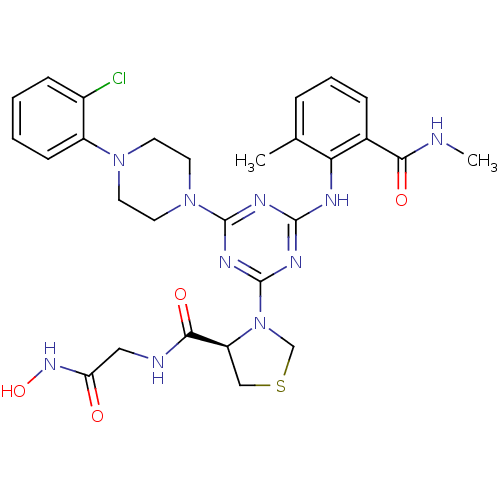 (PDF inhibitor, compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92765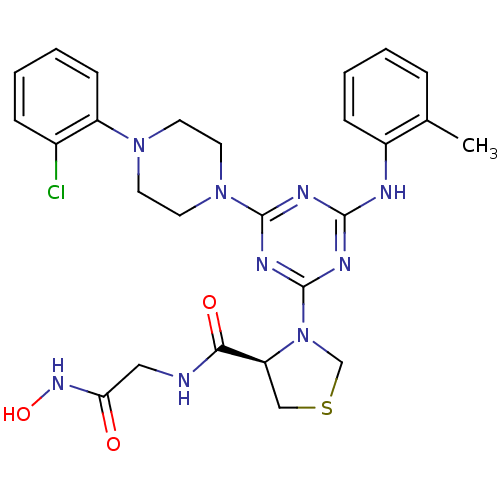 (PDF inhibitor, compound 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169013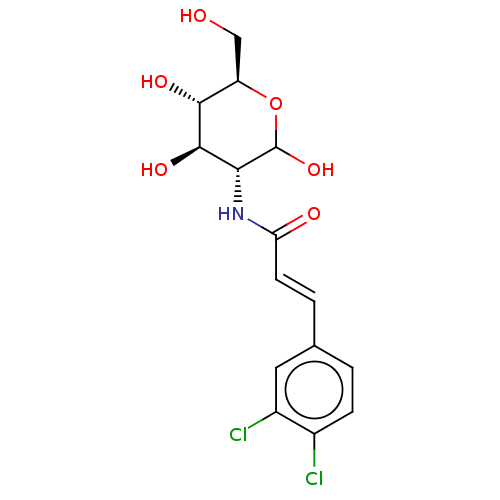 (CHEMBL3805703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) assessed as formation of G6P by continuou... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92759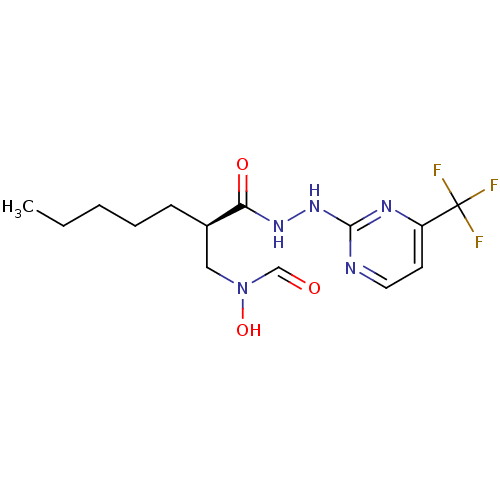 (PDF inhibitor, compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92762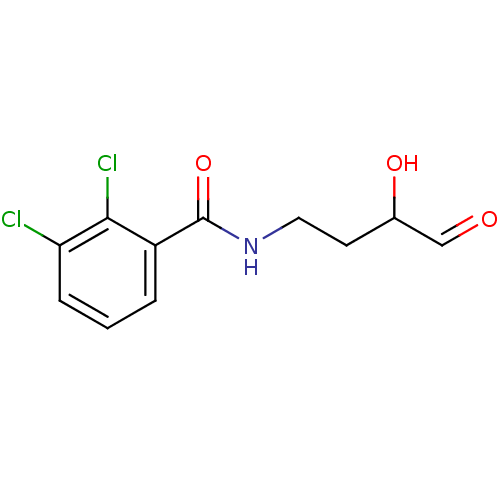 (PDF inhibitor, compound 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92763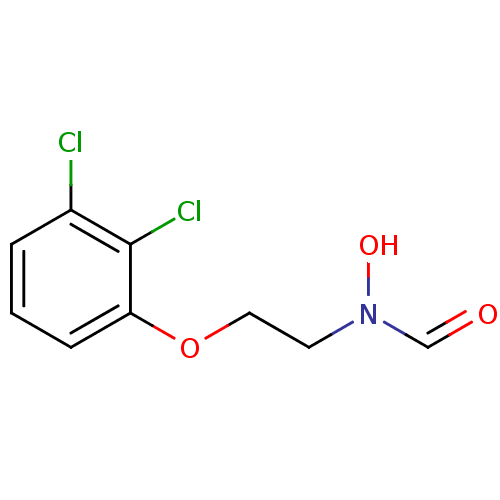 (PDF inhibitor, compound 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118382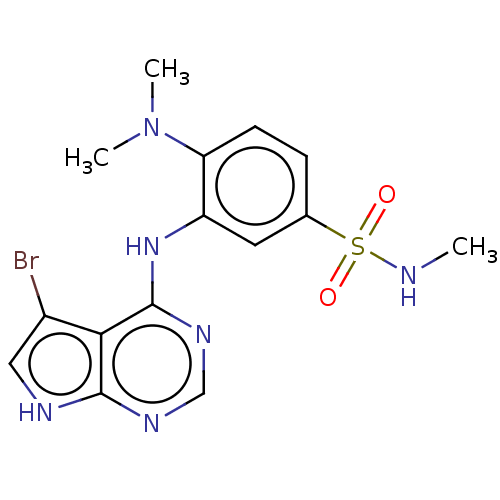 (CHEMBL3613198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451322 (CHEMBL4210026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118381 (CHEMBL3613197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118384 (CHEMBL3613305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435764 (CHEMBL2392692) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451338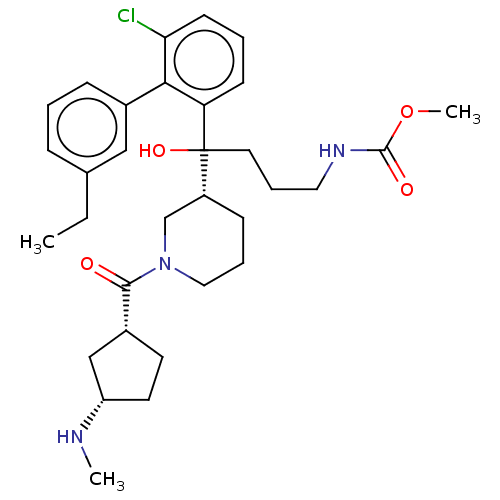 (CHEMBL4207673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118386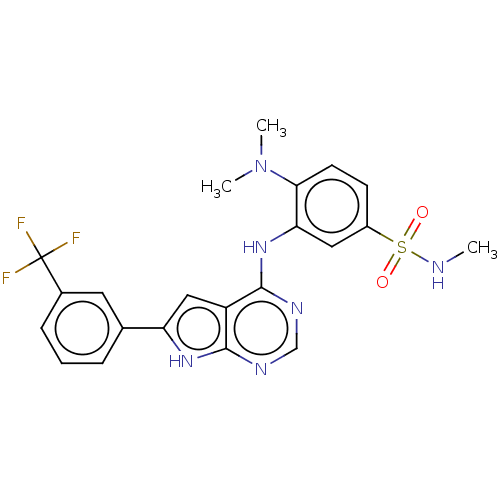 (CHEMBL3613307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451337 (CHEMBL4204601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TNNI3K (Homo sapiens (Human)) | BDBM50118382 (CHEMBL3613198) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human myc-tagged TNNI3K autophosphorylation overexpressed in HEKMSRII cells preincubated for 30 mins followed by pervanadate solution a... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118465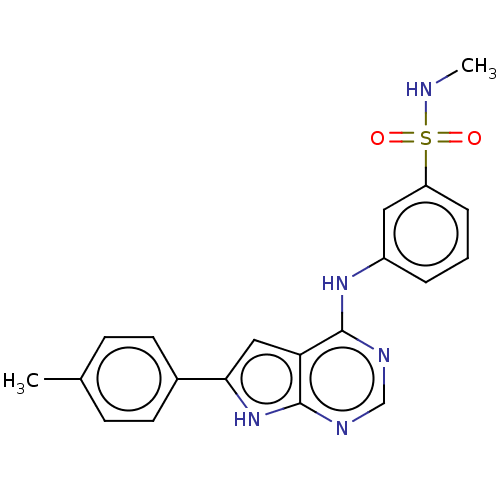 (CHEMBL3613187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118464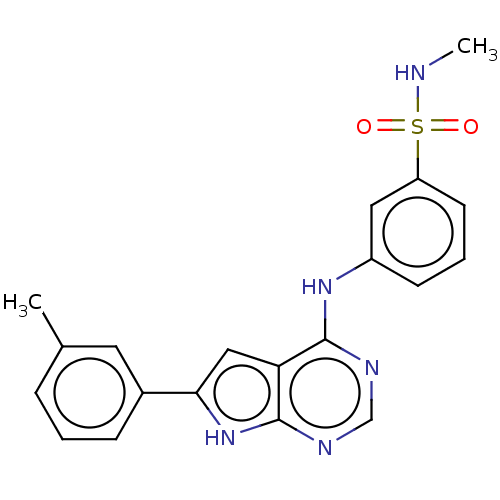 (CHEMBL3613186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118420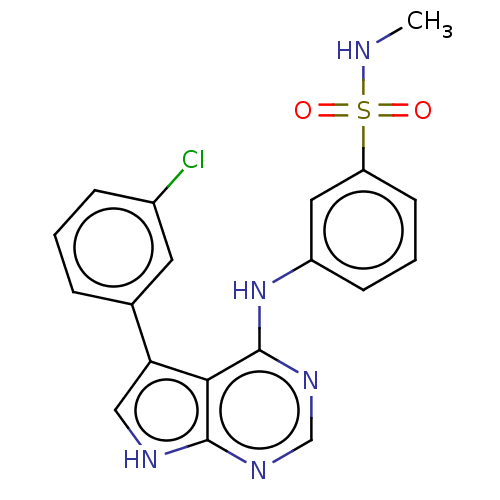 (CHEMBL3613177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118428 (CHEMBL3613184) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435753 (CHEMBL2392698) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435754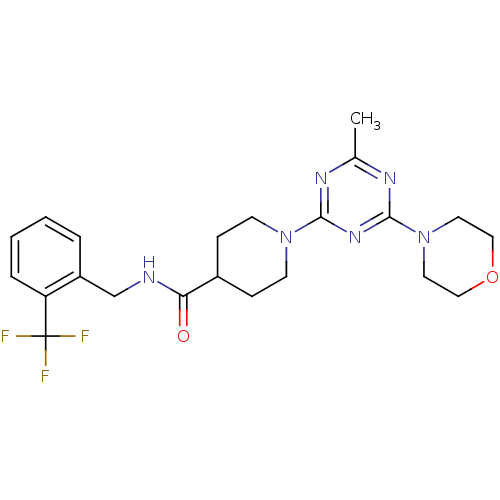 (CHEMBL2392697) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435755 (CHEMBL2392696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435756 (CHEMBL2392695) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435757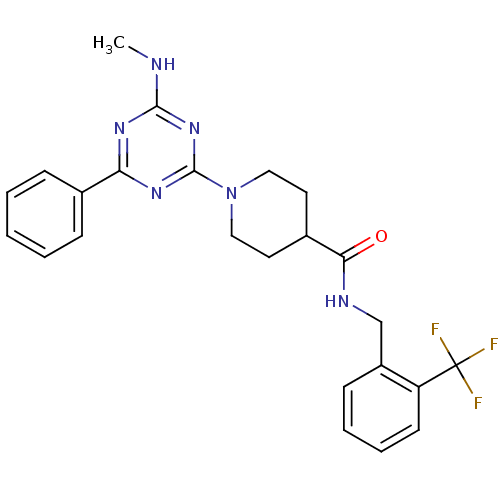 (CHEMBL2392694) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435758 (CHEMBL2392693) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435753 (CHEMBL2392698) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435765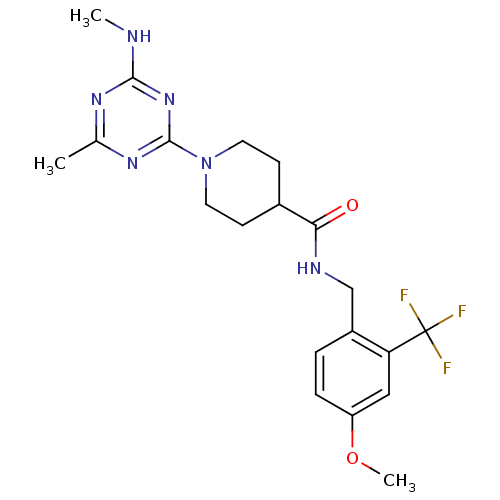 (CHEMBL2392691) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451321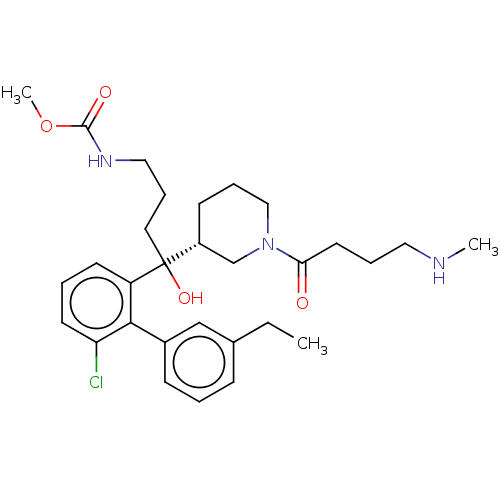 (CHEMBL4211388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118468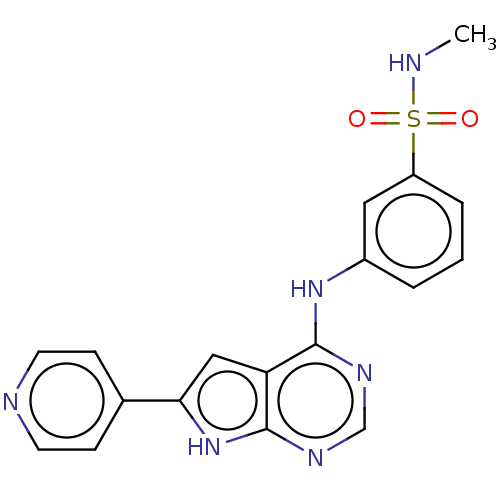 (CHEMBL3613190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451325 (CHEMBL4202424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118467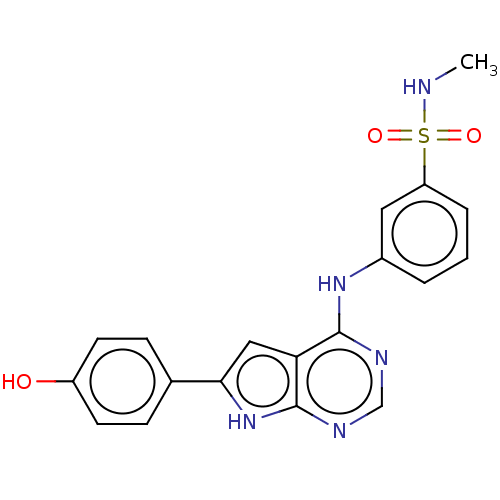 (CHEMBL3613189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435745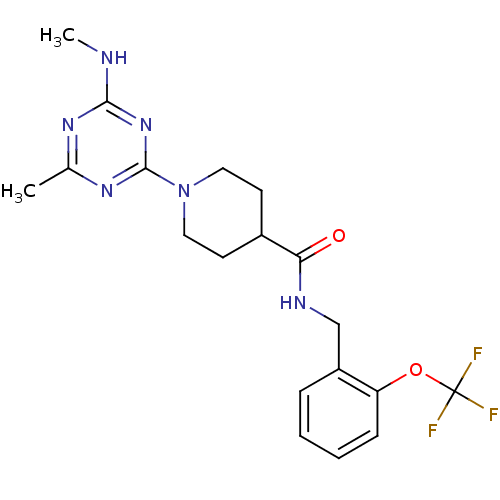 (CHEMBL2392706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451314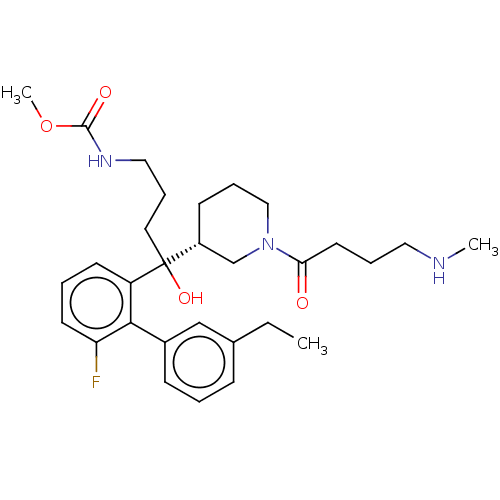 (CHEMBL4207648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118388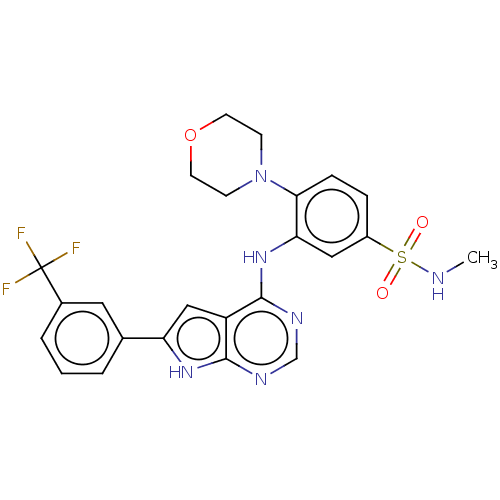 (CHEMBL3613309) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451334 (CHEMBL4213138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50118383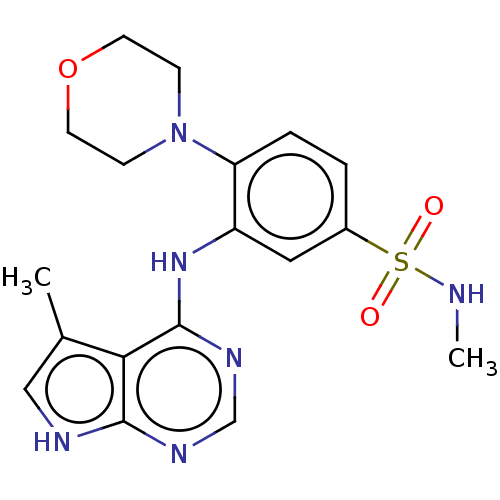 (CHEMBL3613199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His6-tagged full length BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in Baculovirus expression system by BRAMA meth... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TNNI3K (Homo sapiens (Human)) | BDBM50118381 (CHEMBL3613197) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human myc-tagged TNNI3K autophosphorylation overexpressed in HEKMSRII cells preincubated for 30 mins followed by pervanadate solution a... | J Med Chem 58: 7431-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00931 BindingDB Entry DOI: 10.7270/Q2FT8NV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451318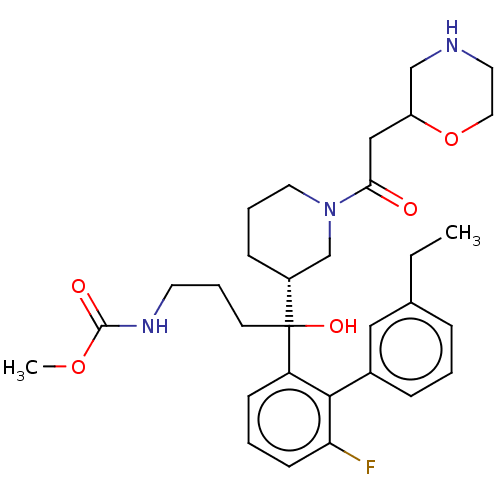 (CHEMBL4218098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451328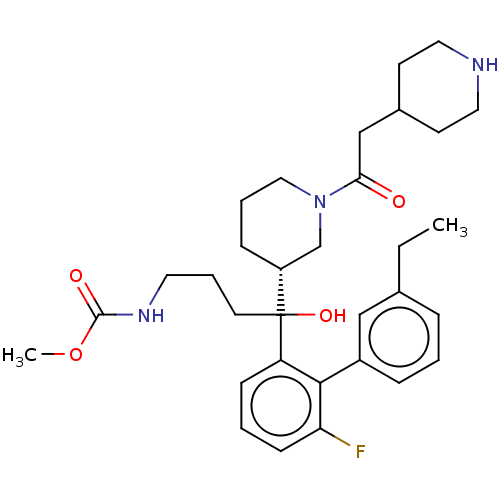 (CHEMBL4211697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451322 (CHEMBL4210026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using angiotensinogen as substrate measured for 90 mins by [125I]-angiotensin based radioimmunoassay | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536168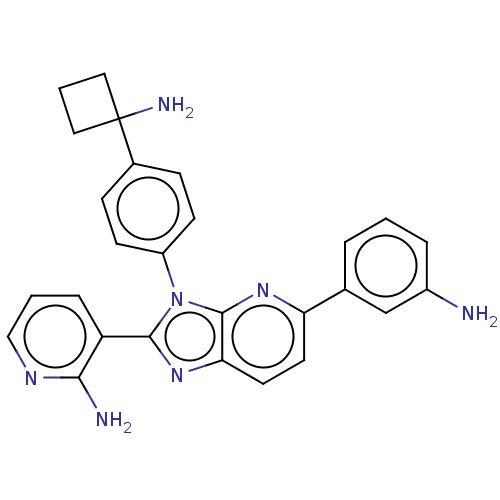 (CHEMBL4574111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 BindingDB Entry DOI: 10.7270/Q2RN3CCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50435764 (CHEMBL2392692) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435765 (CHEMBL2392691) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451331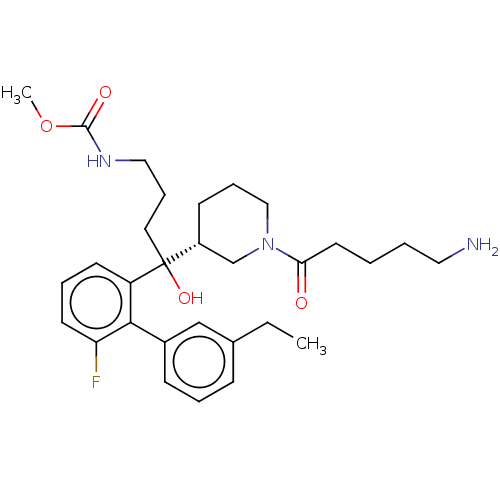 (CHEMBL4217145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451319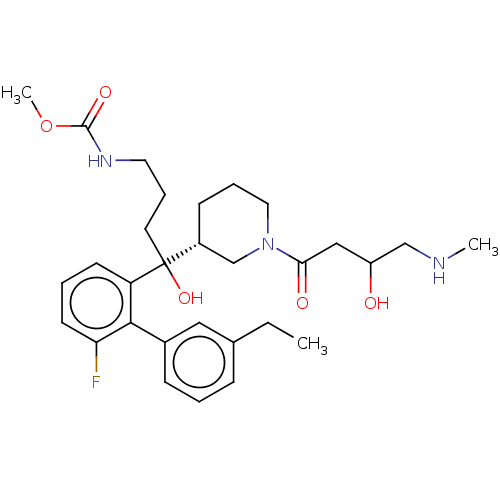 (CHEMBL4212859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451320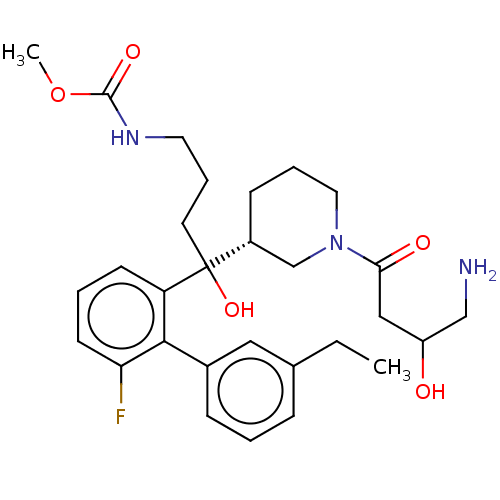 (CHEMBL4216413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451329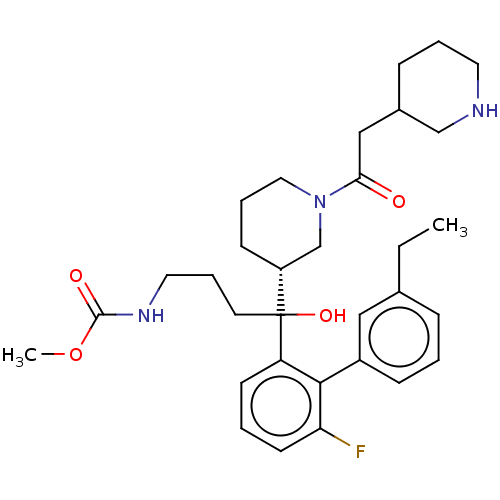 (CHEMBL4212429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 524 total ) | Next | Last >> |