

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Integrase | ||
| Ligand | BDBM50559796 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_2070418 (CHEMBL4725952) | ||
| Ki | 17±n/a nM | ||
| Citation |  Patel, M; Naidu, BN; Dicker, I; Higley, H; Lin, Z; Terry, B; Protack, T; Krystal, M; Jenkins, S; Parker, D; Panja, C; Rampulla, R; Mathur, A; Meanwell, NA; Walker, MA Design, synthesis and SAR study of bridged tricyclic pyrimidinone carboxamides as HIV-1 integrase inhibitors. Bioorg Med Chem28:0 (2020) [PubMed] Article Patel, M; Naidu, BN; Dicker, I; Higley, H; Lin, Z; Terry, B; Protack, T; Krystal, M; Jenkins, S; Parker, D; Panja, C; Rampulla, R; Mathur, A; Meanwell, NA; Walker, MA Design, synthesis and SAR study of bridged tricyclic pyrimidinone carboxamides as HIV-1 integrase inhibitors. Bioorg Med Chem28:0 (2020) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Integrase | |||
| Name: | Integrase | ||
| Synonyms: | Integrase | int | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 32028.36 | ||
| Organism: | Human immunodeficiency virus 1 | ||
| Description: | ChEMBL_118431 | ||
| Residue: | 286 | ||
| Sequence: |
| ||
| BDBM50559796 | |||
| n/a | |||
| Name | BDBM50559796 | ||
| Synonyms: | CHEMBL4779601 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C23H26FN5O5 | ||
| Mol. Mass. | 471.4814 | ||
| SMILES | CN(C)C(=O)C(=O)NC12CCC(CC1)Cn1c2nc(C(=O)NCc2ccc(F)cc2)c(O)c1=O |(8.88,-35.92,;8.94,-37.46,;7.64,-38.28,;10.3,-38.18,;10.37,-39.71,;11.61,-37.35,;11.54,-35.81,;12.97,-38.07,;14.27,-37.24,;15.33,-38.38,;16.87,-38.26,;17.75,-36.98,;16.12,-37.47,;15.67,-36.2,;17.28,-35.51,;15.84,-34.94,;14.5,-35.71,;13.18,-34.95,;13.18,-33.41,;11.84,-32.65,;11.83,-31.11,;10.51,-33.42,;9.17,-32.65,;7.84,-33.43,;6.51,-32.66,;5.18,-33.43,;5.18,-34.97,;3.85,-35.75,;6.52,-35.74,;7.85,-34.96,;14.5,-32.64,;14.5,-31.1,;15.83,-33.41,;17.17,-32.65,)| | ||
| Structure | 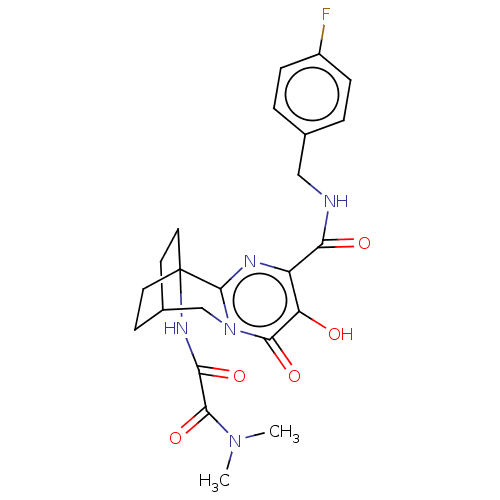
| ||