

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122692 (CHEMBL282336 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122706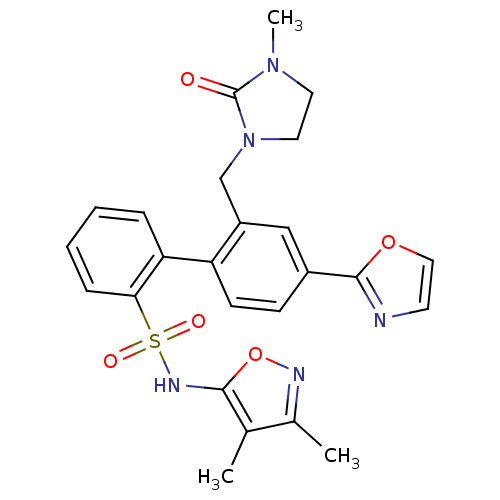 (2'-(3-Methyl-2-oxo-imidazolidin-1-ylmethyl)-4'-oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122693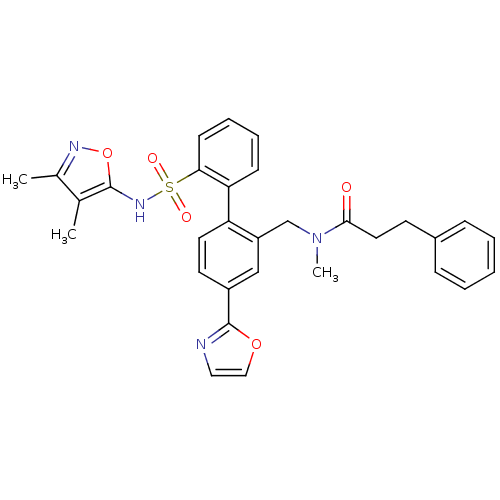 (CHEMBL29346 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122686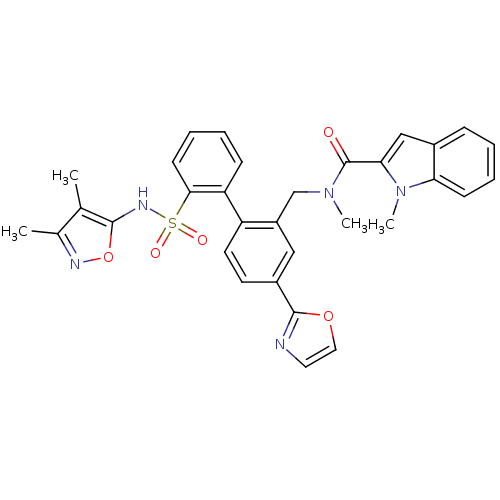 (1-Methyl-1H-indole-2-carboxylic acid [2'-(3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122694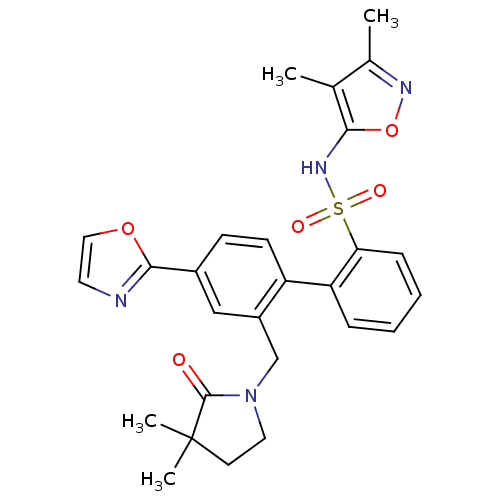 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122715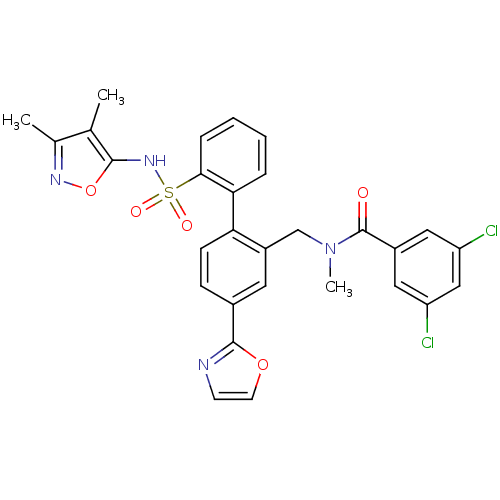 (3,5-Dichloro-N-[2'-(3,4-dimethyl-isoxazol-5-ylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122676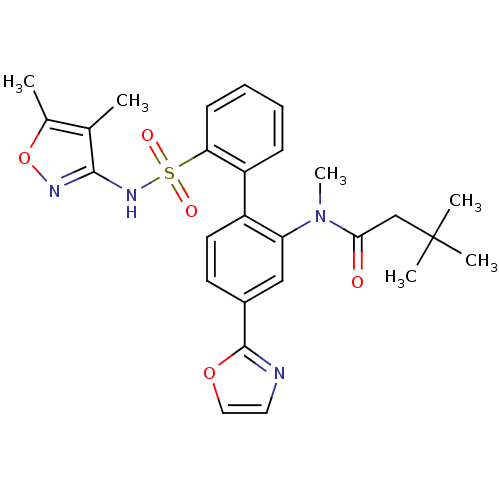 (CHEMBL274489 | N-[2'-(4,5-Dimethyl-isoxazol-3-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122712 (CHEMBL440780 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122700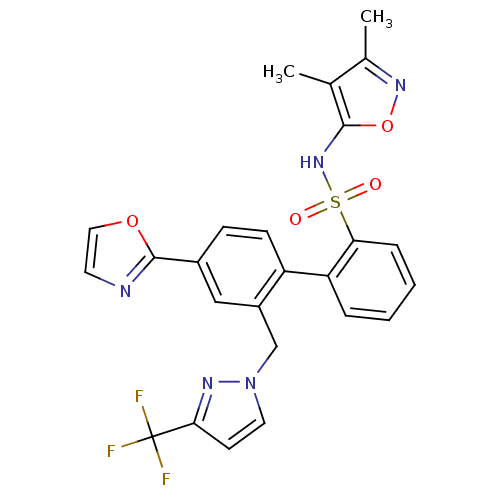 (4'-Oxazol-2-yl-2'-(3-trifluoromethyl-pyrazol-1-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122707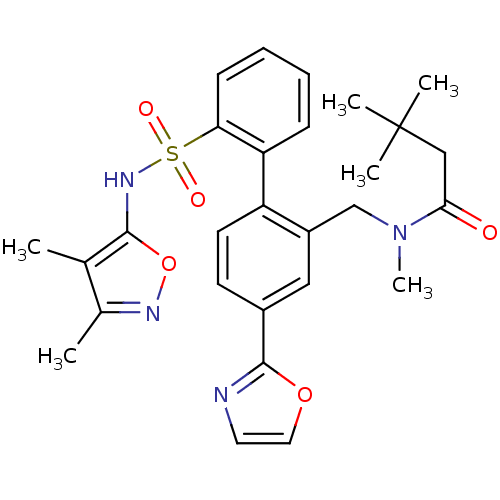 (CHEMBL281659 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122697 (2'-[(Methyl-phenyl-amino)-methyl]-4'-oxazol-2-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122681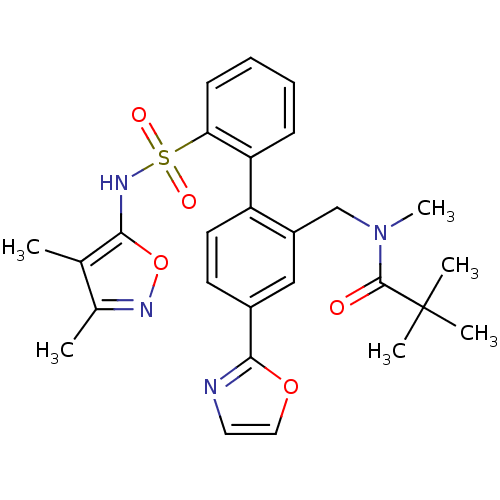 (CHEMBL27855 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122713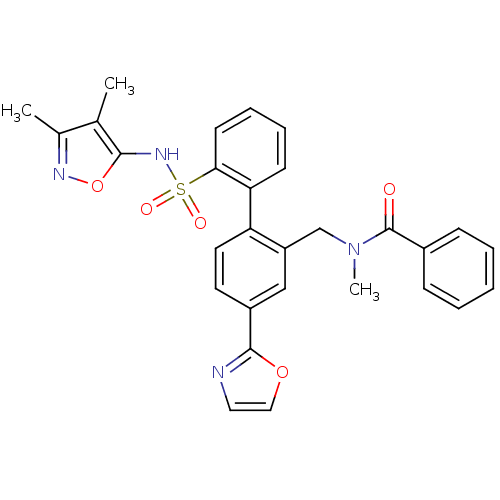 (CHEMBL282359 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122698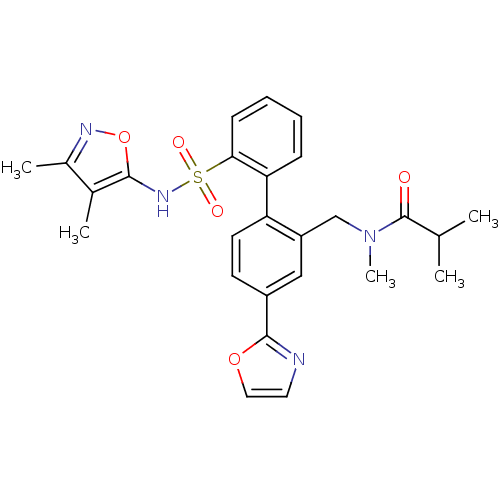 (CHEMBL28863 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122684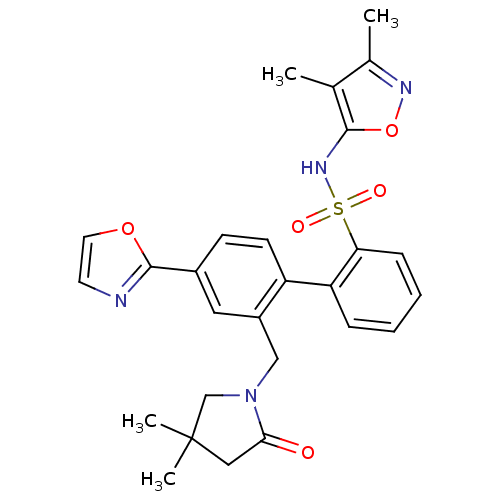 (2'-(4,4-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122690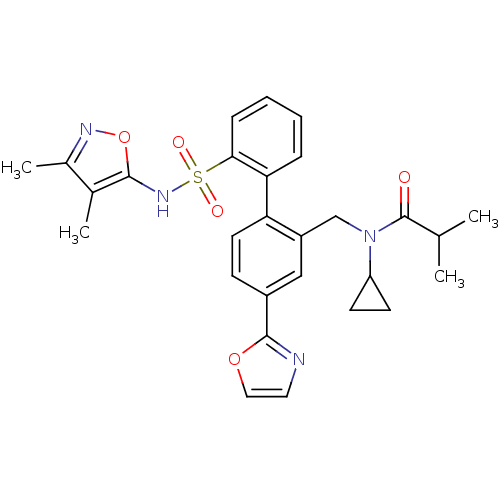 (CHEMBL28963 | N-Cyclopropyl-N-[2'-(3,4-dimethyl-is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122696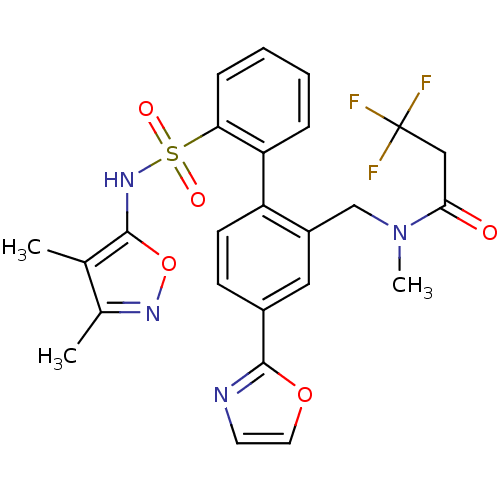 (CHEMBL281549 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582801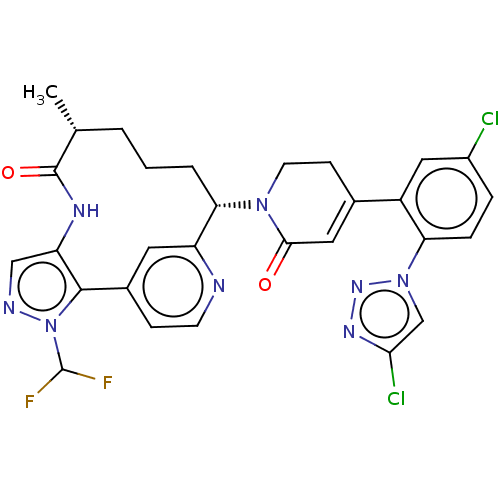 (CHEMBL5076656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250493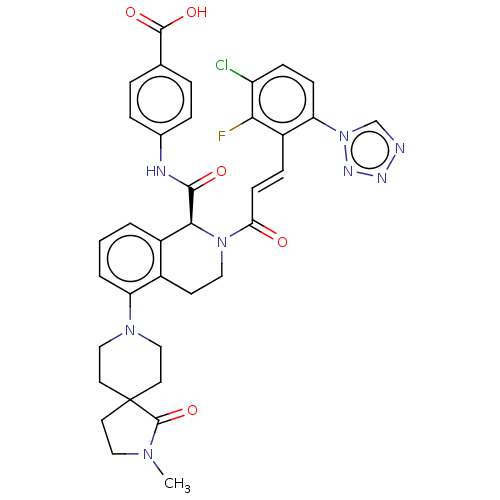 (CHEMBL4068445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122703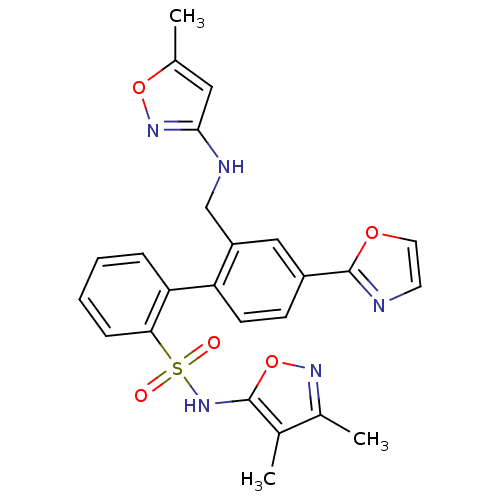 (2'-[(5-Methyl-isoxazol-3-ylamino)-methyl]-4'-oxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250492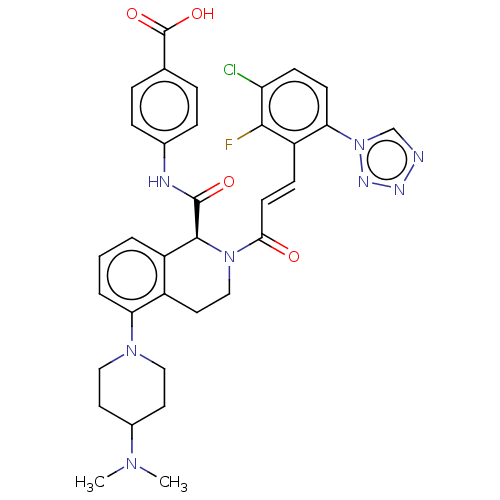 (CHEMBL4097304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247411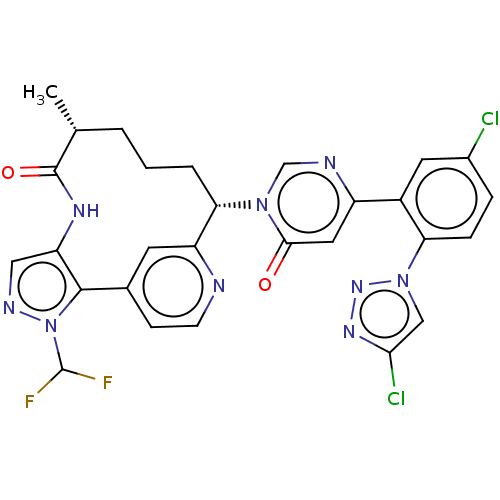 (US10336754, Example 353 | US11053247, Example 353 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582799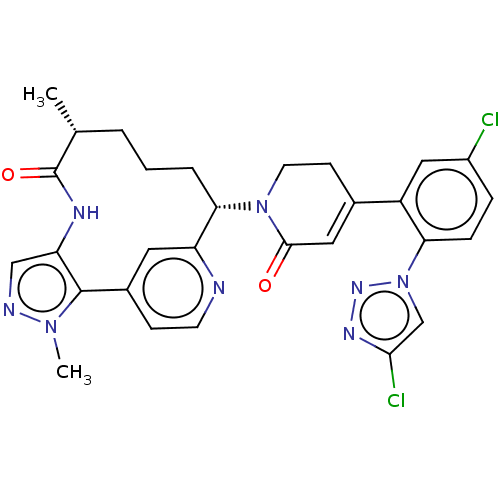 (CHEMBL5094166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122711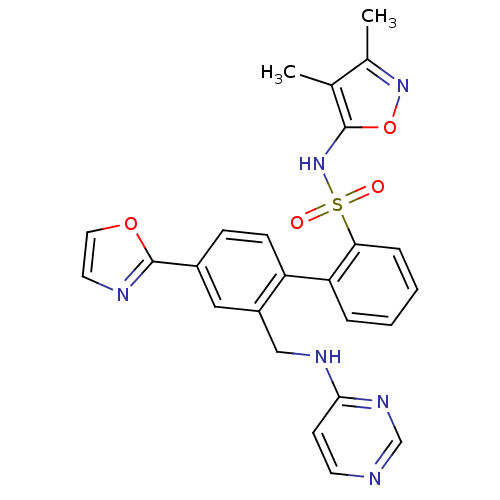 (4'-Oxazol-2-yl-2'-(pyrimidin-4-ylaminomethyl)-biph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122704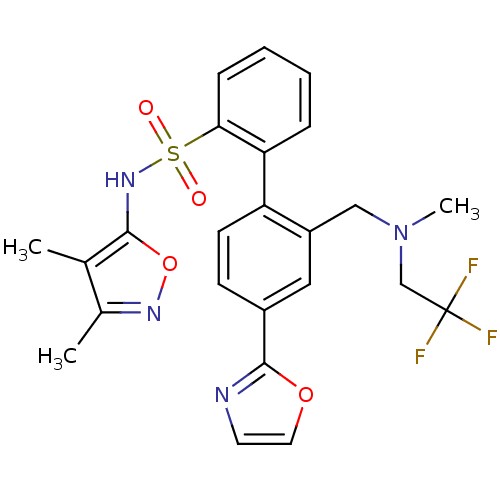 (2'-{[Methyl-(2,2,2-trifluoro-ethyl)-amino]-methyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582800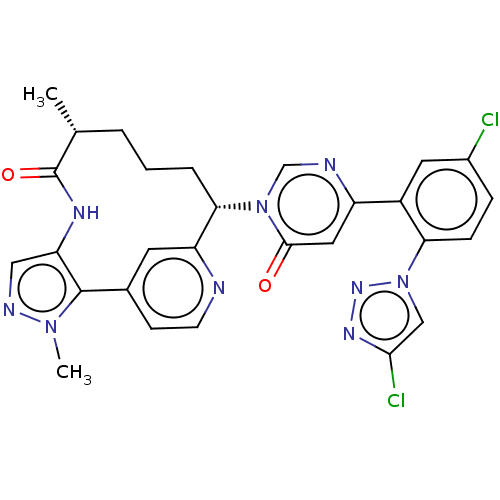 (CHEMBL5093567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514438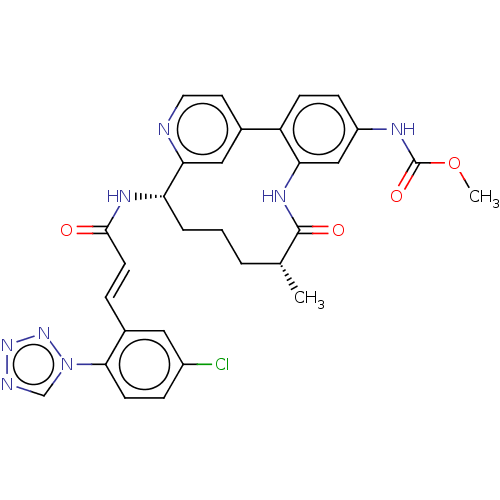 (CHEMBL4439729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269186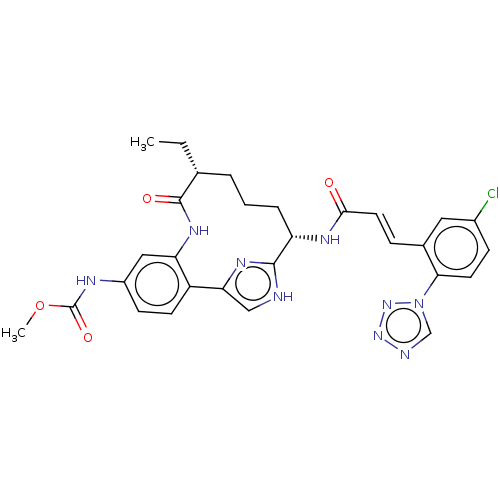 (CHEMBL4089185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122678 (2'-Isobutoxymethyl-4'-oxazol-2-yl-biphenyl-2-sulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091104 (CHEMBL29422 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50448583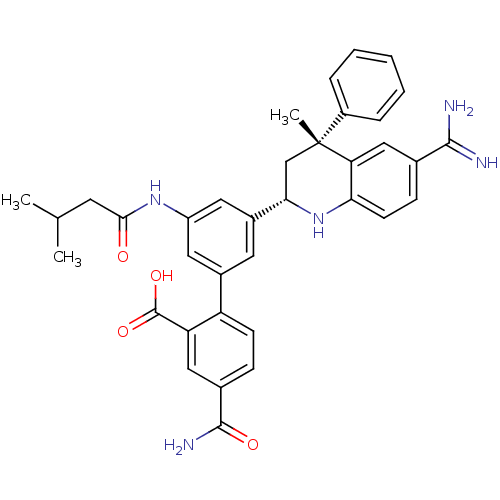 (CHEMBL3127491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514437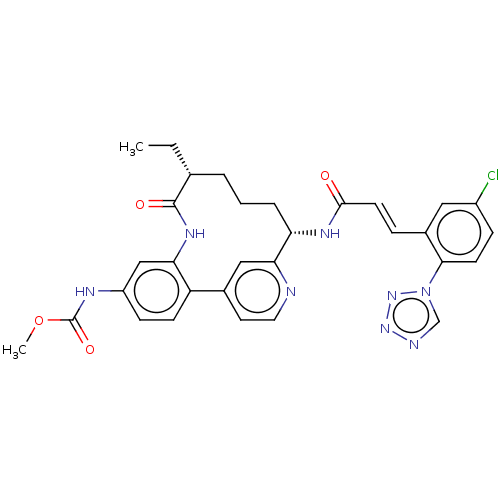 (CHEMBL4444690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091104 (CHEMBL29422 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Human Endothelin A receptor expressed in CHO Cells. | J Med Chem 43: 3111-7 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122710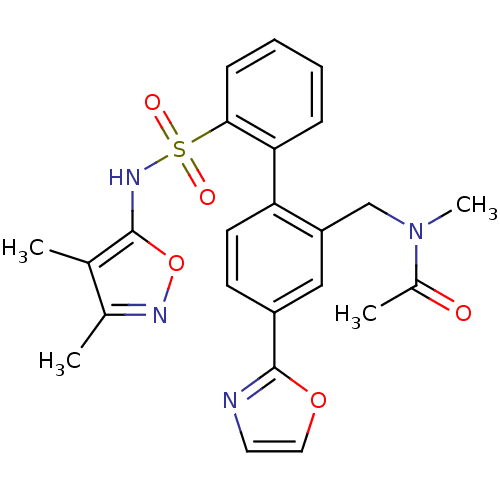 (CHEMBL284656 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586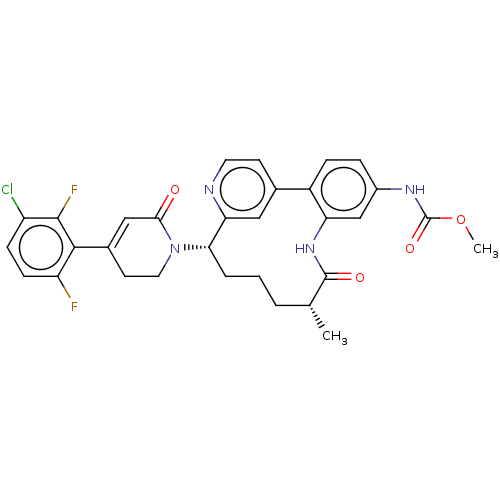 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032873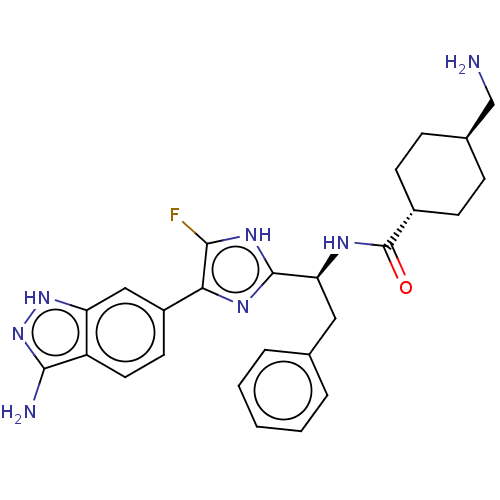 (CHEMBL3355684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122699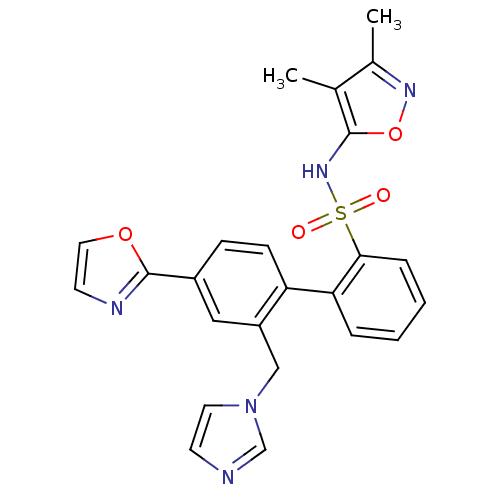 (2'-Imidazol-1-ylmethyl-4'-oxazol-2-yl-biphenyl-2-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122680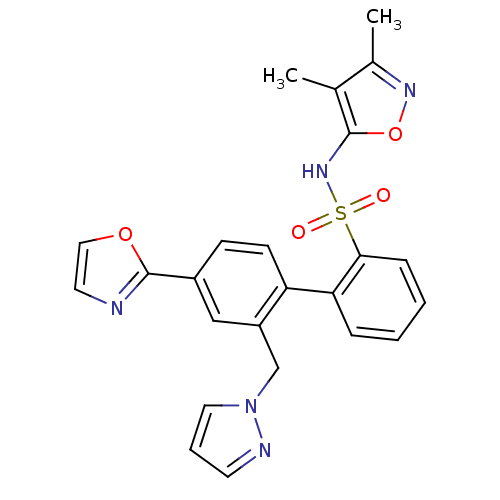 (4'-Oxazol-2-yl-2'-pyrazol-1-ylmethyl-biphenyl-2-su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032874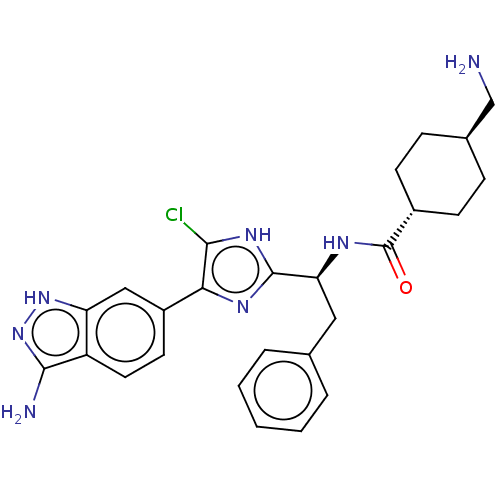 (CHEMBL3355683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250501 (CHEMBL4078562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269195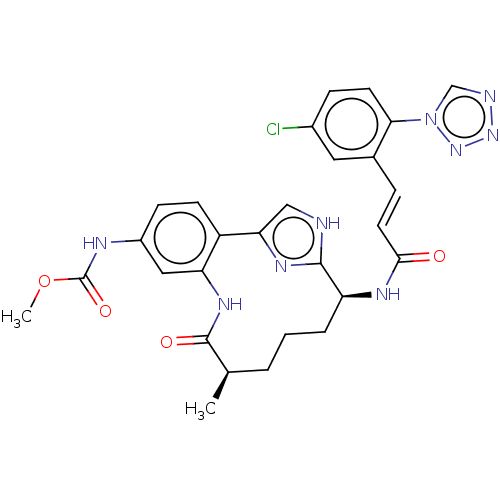 (CHEMBL4101766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122689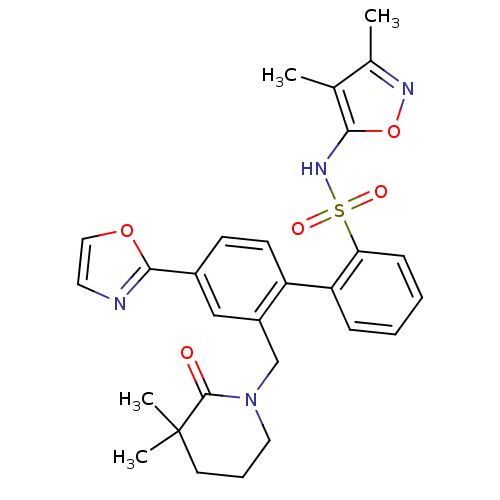 (2'-(3,3-Dimethyl-2-oxo-piperidin-1-ylmethyl)-4'-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122679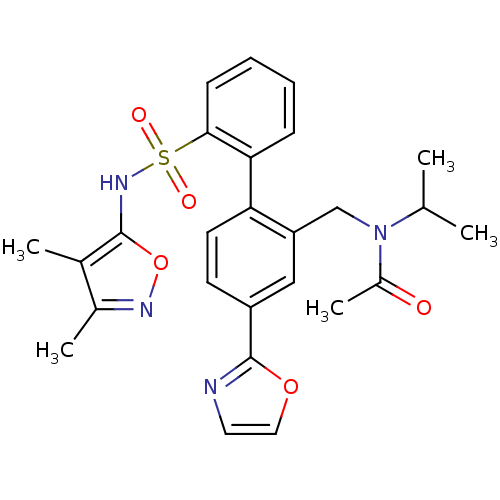 (CHEMBL29775 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122705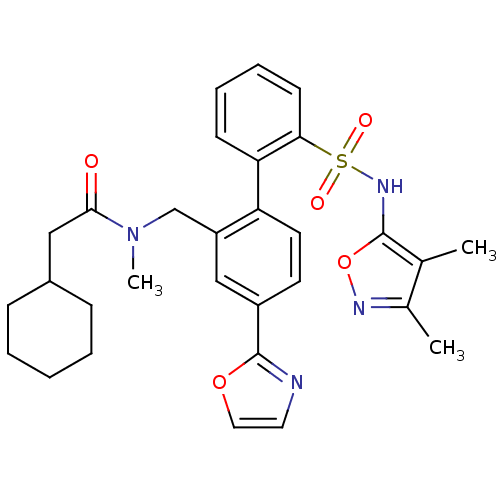 (2-Cyclohexyl-N-[2'-(3,4-dimethyl-isoxazol-5-ylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250490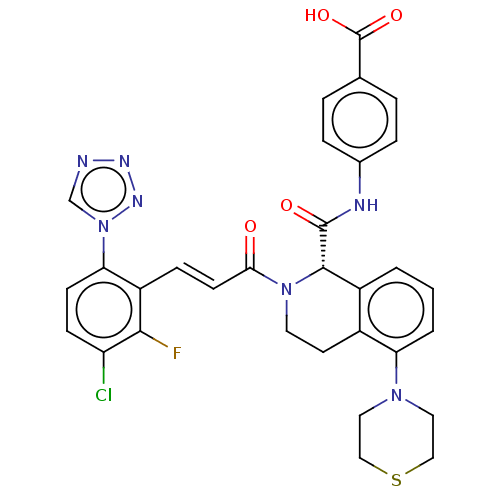 (CHEMBL4087166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541581 (CHEMBL4646341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091101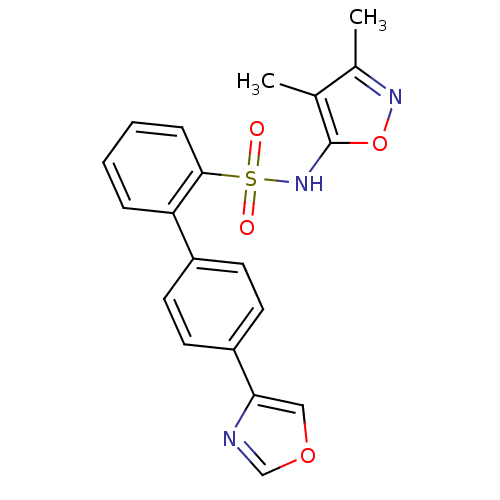 (4'-Oxazol-4-yl-biphenyl-2-sulfonic acid (3,4-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Human Endothelin A receptor expressed in CHO Cells. | J Med Chem 43: 3111-7 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122695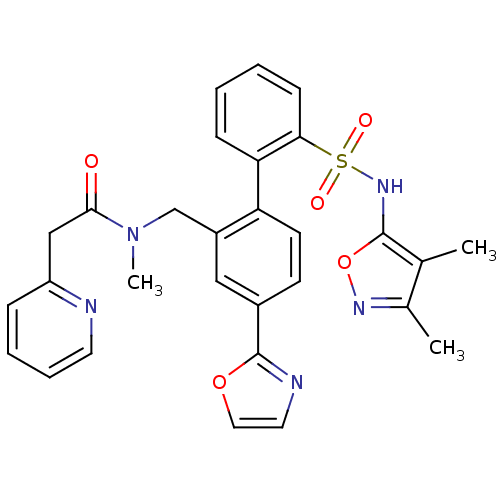 (CHEMBL29710 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541577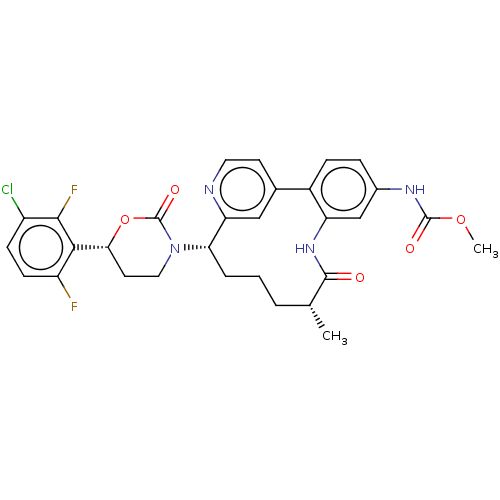 (CHEMBL4646441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3808 total ) | Next | Last >> |