| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Matrix metalloproteinase-14 |
|---|
| Ligand | BDBM50213416 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_447908 (CHEMBL898158) |
|---|
| IC50 | 2490±n/a nM |
|---|
| Citation |  Moroy, G; Denhez, C; El Mourabit, H; Toribio, A; Dassonville, A; Decarme, M; Renault, JH; Mirand, C; Bellon, G; Sapi, J; Alix, AJ; Hornebeck, W; Bourguet, E Simultaneous presence of unsaturation and long alkyl chain at P'1 of Ilomastat confers selectivity for gelatinase A (MMP-2) over gelatinase B (MMP-9) inhibition as shown by molecular modelling studies. Bioorg Med Chem15:4753-66 (2007) [PubMed] Article Moroy, G; Denhez, C; El Mourabit, H; Toribio, A; Dassonville, A; Decarme, M; Renault, JH; Mirand, C; Bellon, G; Sapi, J; Alix, AJ; Hornebeck, W; Bourguet, E Simultaneous presence of unsaturation and long alkyl chain at P'1 of Ilomastat confers selectivity for gelatinase A (MMP-2) over gelatinase B (MMP-9) inhibition as shown by molecular modelling studies. Bioorg Med Chem15:4753-66 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Matrix metalloproteinase-14 |
|---|
| Name: | Matrix metalloproteinase-14 |
|---|
| Synonyms: | MMP-14 | MMP-X1 | MMP14 | MMP14_HUMAN | MT-MMP 1 | MT1-MMP | MT1MMP | MTMMP1 | Matrix Metalloproteinase-14 (MMP-14) | Matrix metalloproteinase 14 | Matrix metalloproteinase-14 | Matrix metalloproteinase-14 (MMP14) | Membrane-type matrix metalloproteinase 1 | Membrane-type-1 matrix metalloproteinase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 65900.19 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P50281 |
|---|
| Residue: | 582 |
|---|
| Sequence: | MSPAPRPPRCLLLPLLTLGTALASLGSAQSSSFSPEAWLQQYGYLPPGDLRTHTQRSPQS
LSAAIAAMQKFYGLQVTGKADADTMKAMRRPRCGVPDKFGAEIKANVRRKRYAIQGLKWQ
HNEITFCIQNYTPKVGEYATYEAIRKAFRVWESATPLRFREVPYAYIREGHEKQADIMIF
FAEGFHGDSTPFDGEGGFLAHAYFPGPNIGGDTHFDSAEPWTVRNEDLNGNDIFLVAVHE
LGHALGLEHSSDPSAIMAPFYQWMDTENFVLPDDDRRGIQQLYGGESGFPTKMPPQPRTT
SRPSVPDKPKNPTYGPNICDGNFDTVAMLRGEMFVFKERWFWRVRNNQVMDGYPMPIGQF
WRGLPASINTAYERKDGKFVFFKGDKHWVFDEASLEPGYPKHIKELGRGLPTDKIDAALF
WMPNGKTYFFRGNKYYRFNEELRAVDSEYPKNIKVWEGIPESPRGSFMGSDEVFTYFYKG
NKYWKFNNQKLKVEPGYPKSALRDWMGCPSGGRPDEGTEEETEVIIIEVDEEGGGAVSAA
AVVLPVLLLLLVLAVGLAVFFFRRHGTPRRLLYCQRSLLDKV
|
|
|
|---|
| BDBM50213416 |
|---|
| n/a |
|---|
| Name | BDBM50213416 |
|---|
| Synonyms: | (2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2-en-1-ylidene]propionyl-L-tryptophan-N-methylamide | CHEMBL391509 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H26N4O4 |
|---|
| Mol. Mass. | 446.4983 |
|---|
| SMILES | CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| |
|---|
| Structure | 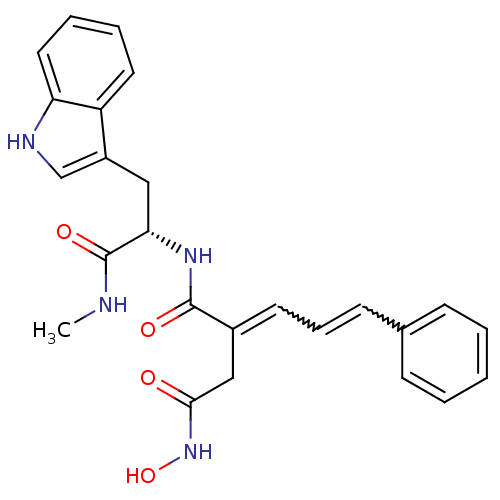
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Moroy, G; Denhez, C; El Mourabit, H; Toribio, A; Dassonville, A; Decarme, M; Renault, JH; Mirand, C; Bellon, G; Sapi, J; Alix, AJ; Hornebeck, W; Bourguet, E Simultaneous presence of unsaturation and long alkyl chain at P'1 of Ilomastat confers selectivity for gelatinase A (MMP-2) over gelatinase B (MMP-9) inhibition as shown by molecular modelling studies. Bioorg Med Chem15:4753-66 (2007) [PubMed] Article
Moroy, G; Denhez, C; El Mourabit, H; Toribio, A; Dassonville, A; Decarme, M; Renault, JH; Mirand, C; Bellon, G; Sapi, J; Alix, AJ; Hornebeck, W; Bourguet, E Simultaneous presence of unsaturation and long alkyl chain at P'1 of Ilomastat confers selectivity for gelatinase A (MMP-2) over gelatinase B (MMP-9) inhibition as shown by molecular modelling studies. Bioorg Med Chem15:4753-66 (2007) [PubMed] Article