| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50264161 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_535290 (CHEMBL982615) |
|---|
| IC50 | 6800±n/a nM |
|---|
| Citation |  Sato, I; Morihira, K; Inami, H; Kubota, H; Morokata, T; Suzuki, K; Iura, Y; Nitta, A; Imaoka, T; Takahashi, T; Takeuchi, M; Ohta, M; Tsukamoto, S Design and synthesis of 6-fluoro-2-naphthyl derivatives as novel CCR3 antagonists with reduced CYP2D6 inhibition. Bioorg Med Chem16:8607-18 (2008) [PubMed] Article Sato, I; Morihira, K; Inami, H; Kubota, H; Morokata, T; Suzuki, K; Iura, Y; Nitta, A; Imaoka, T; Takahashi, T; Takeuchi, M; Ohta, M; Tsukamoto, S Design and synthesis of 6-fluoro-2-naphthyl derivatives as novel CCR3 antagonists with reduced CYP2D6 inhibition. Bioorg Med Chem16:8607-18 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50264161 |
|---|
| n/a |
|---|
| Name | BDBM50264161 |
|---|
| Synonyms: | CHEMBL488929 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)methyl]-8-azabicyclo[3.2.1]oct-3-yl}-3-(piperidin-1-ylcarbonyl)isonicotinamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H33FN4O2 |
|---|
| Mol. Mass. | 500.607 |
|---|
| SMILES | Fc1ccc2cc(CN3[C@@H]4CC[C@@H]3CC(C4)NC(=O)c3ccncc3C(=O)N3CCCCC3)ccc2c1 |r,TLB:7:8:14.15.13:10.11,THB:16:14:8:10.11| |
|---|
| Structure | 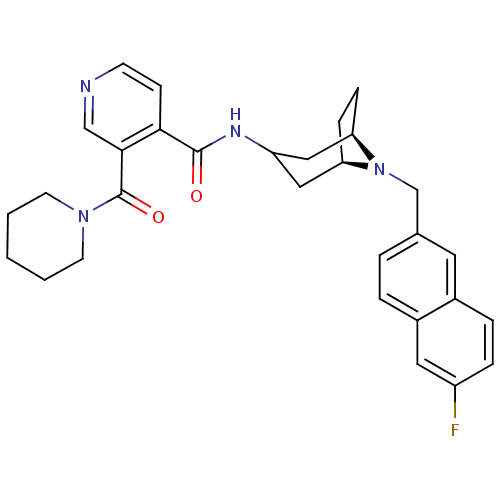
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sato, I; Morihira, K; Inami, H; Kubota, H; Morokata, T; Suzuki, K; Iura, Y; Nitta, A; Imaoka, T; Takahashi, T; Takeuchi, M; Ohta, M; Tsukamoto, S Design and synthesis of 6-fluoro-2-naphthyl derivatives as novel CCR3 antagonists with reduced CYP2D6 inhibition. Bioorg Med Chem16:8607-18 (2008) [PubMed] Article
Sato, I; Morihira, K; Inami, H; Kubota, H; Morokata, T; Suzuki, K; Iura, Y; Nitta, A; Imaoka, T; Takahashi, T; Takeuchi, M; Ohta, M; Tsukamoto, S Design and synthesis of 6-fluoro-2-naphthyl derivatives as novel CCR3 antagonists with reduced CYP2D6 inhibition. Bioorg Med Chem16:8607-18 (2008) [PubMed] Article