

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057433 (CHEMBL278806 | [2-(5-Fluoro-chroman-8-yloxy)-ethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50108501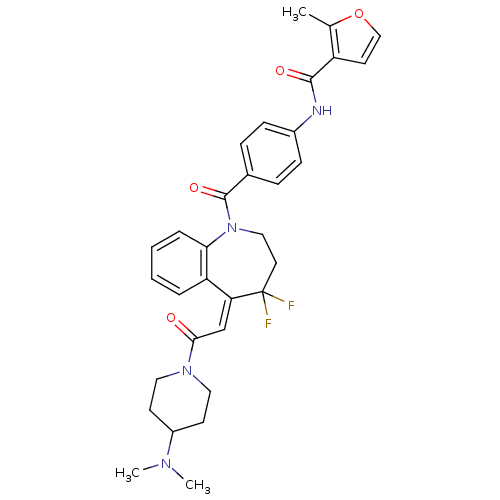 (2-Methyl-furan-3-carboxylic acid (4-{5-[2-(4-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand | Bioorg Med Chem Lett 12: 229-32 (2001) BindingDB Entry DOI: 10.7270/Q2R78FQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057432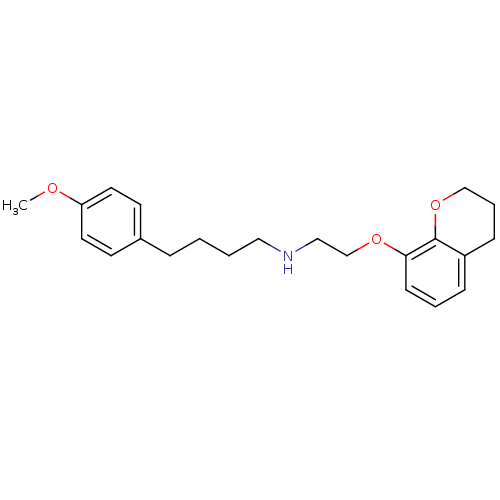 (CHEMBL25215 | [2-(Chroman-8-yloxy)-ethyl]-[4-(4-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065578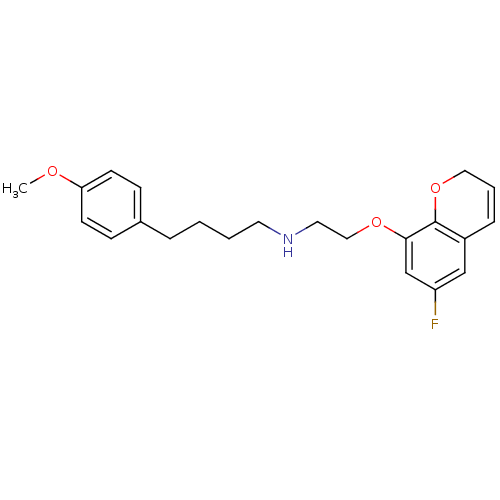 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50108498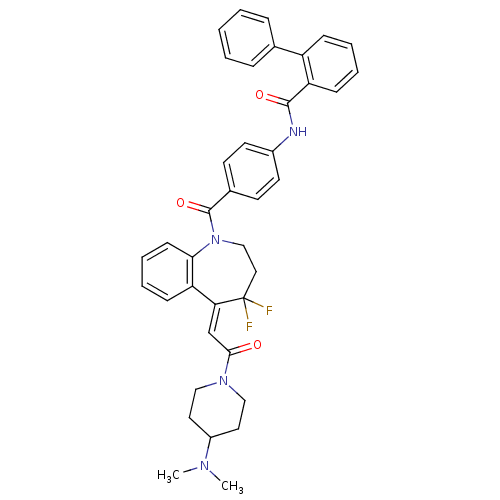 (Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50454212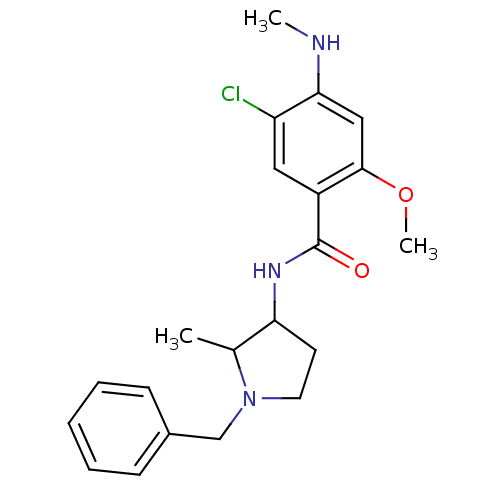 (CHEBI:64217 | Emonapride | Nemonapride) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50454212 (CHEBI:64217 | Emonapride | Nemonapride) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065555 (CHEMBL96578 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375457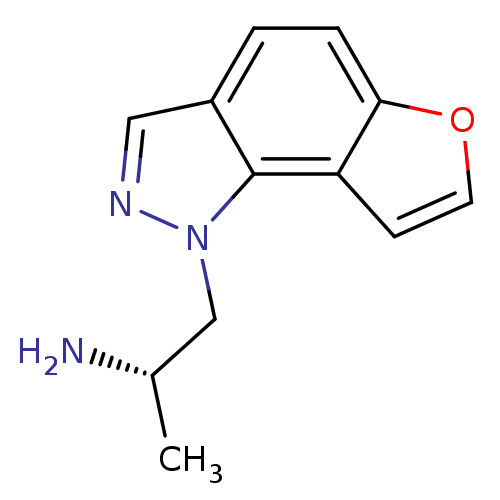 (CHEMBL261476) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375451 (CHEMBL408579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052196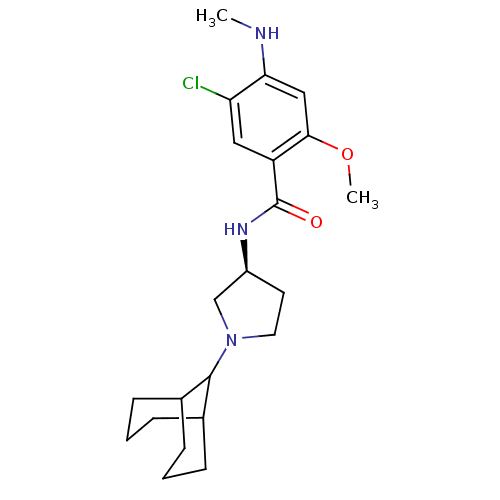 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057434 (8-{2-[4-(4-Methoxy-phenyl)-butylamino]-ethoxy}-chr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065578 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057429 (CHEMBL22328 | [2-(7-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35709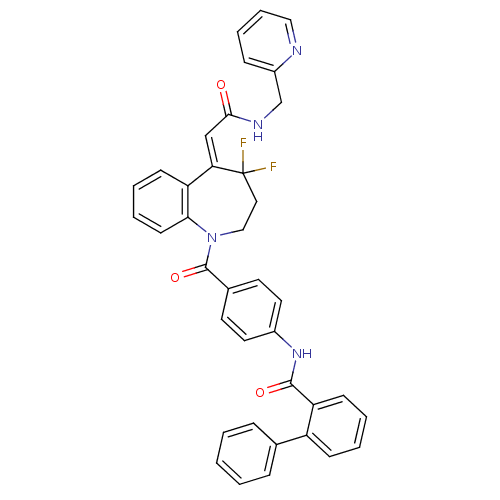 (YM-35278) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Astellas Pharma Inc. | Assay Description The affinities of test compounds for human V2 receptor were evaluated by the radioligand binding study using membrane fractions isolated from CHO cel... | Bioorg Med Chem 16: 9524-35 (2008) Article DOI: 10.1016/j.bmc.2008.09.039 BindingDB Entry DOI: 10.7270/Q2125R12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057428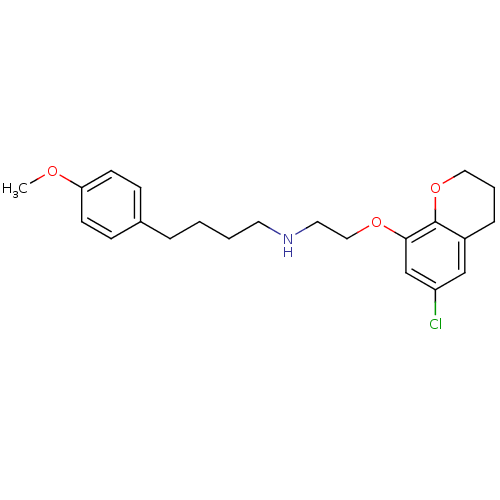 (CHEMBL22682 | [2-(6-Chloro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375452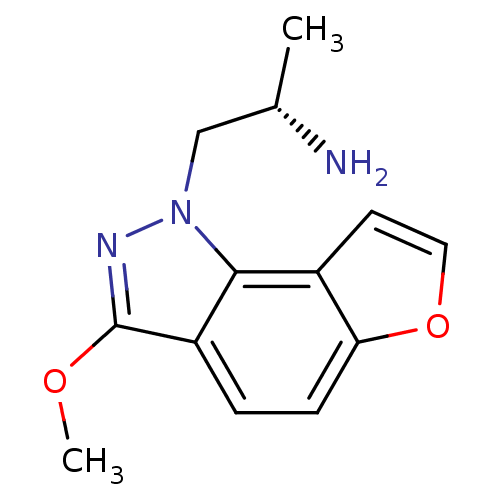 (CHEMBL262092) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50114031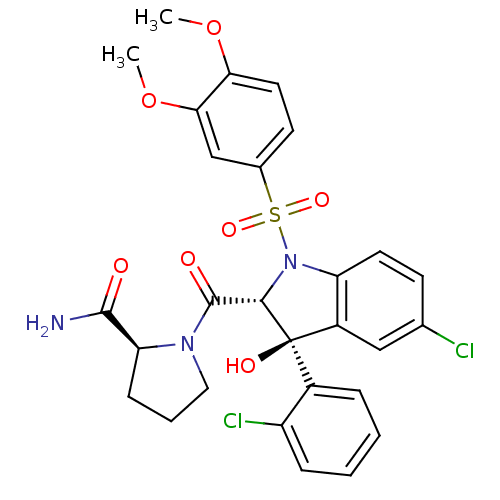 ((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052194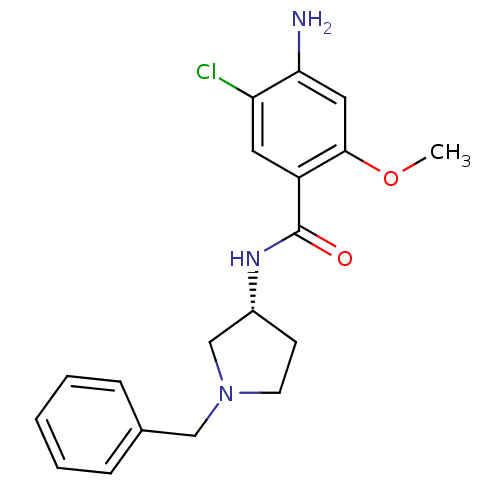 (4-Amino-N-((R)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057432 (CHEMBL25215 | [2-(Chroman-8-yloxy)-ethyl]-[4-(4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated against Dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand; ND = Not determined | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50108498 (Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand | Bioorg Med Chem Lett 12: 229-32 (2001) BindingDB Entry DOI: 10.7270/Q2R78FQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50108498 (Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052196 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563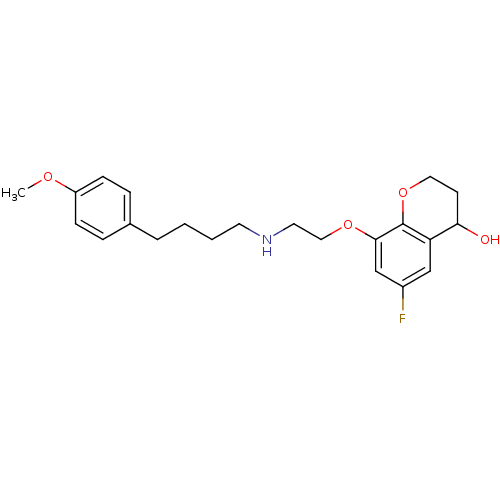 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50108498 (Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at Vasopressin V2 receptor, performed using [3H]-AVP on rat kidney | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057431 (CHEMBL21475 | [4-(4-Methoxy-phenyl)-butyl]-[2-(6-m...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50375457 (CHEMBL261476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2A receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50375451 (CHEMBL408579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2A receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052196 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375450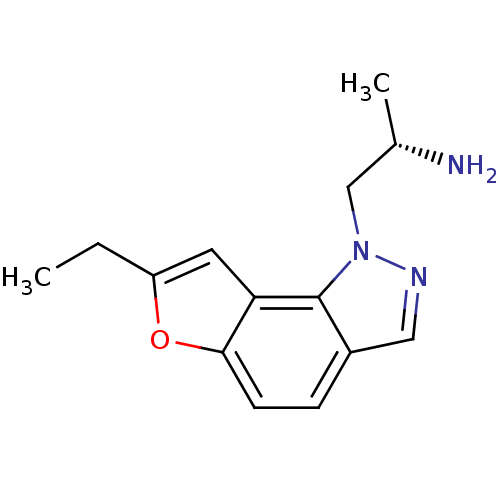 (CHEMBL407909 | YM-348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375450 (CHEMBL407909 | YM-348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 3309-20 (2008) Article DOI: 10.1016/j.bmc.2007.12.009 BindingDB Entry DOI: 10.7270/Q2PN96H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50114033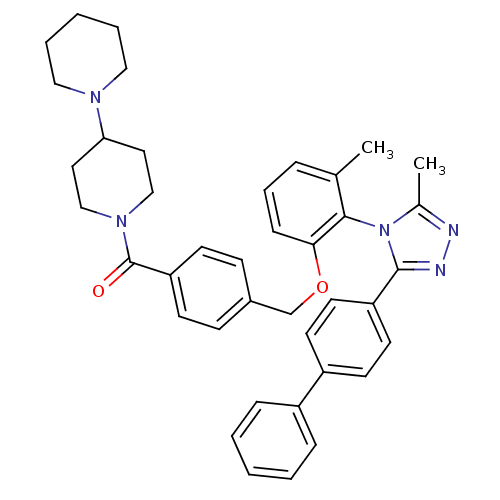 (CHEMBL86667 | {4-[2-(3-Biphenyl-4-yl-5-methyl-[1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50114028 (1-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50114034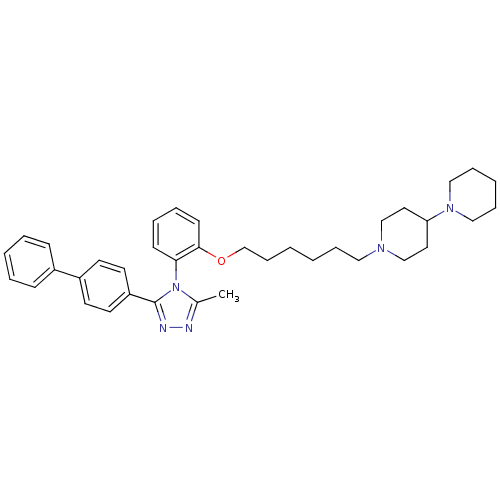 (1'-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50108498 (Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at cloned human Vasopressin V2 receptor stably expressed in CHO cells, using [3H]-AVP as radioligand | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052194 (4-Amino-N-((R)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1340 total ) | Next | Last >> |