| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM254160 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1616389 (CHEMBL3858458) |
|---|
| IC50 | 2200±n/a nM |
|---|
| Citation |  Swanson, DM; Savall, BM; Coe, KJ; Schoetens, F; Koudriakova, T; Skaptason, J; Wall, J; Rech, J; Deng, X; De Angelis, M; Everson, A; Lord, B; Wang, Q; Ao, H; Scott, B; Sepassi, K; Lovenberg, TW; Carruthers, NI; Bhattacharya, A; Letavic, MA Identification of (R)-(2-Chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyridin-2-yl)-4-methyl-6,7-dihydro-1H-imidazo[4,5-c]pyridin-5(4H)-yl)methanone (JNJ 54166060), a Small Molecule Antagonist of the P2X7 receptor. J Med Chem59:8535-48 (2016) [PubMed] Article Swanson, DM; Savall, BM; Coe, KJ; Schoetens, F; Koudriakova, T; Skaptason, J; Wall, J; Rech, J; Deng, X; De Angelis, M; Everson, A; Lord, B; Wang, Q; Ao, H; Scott, B; Sepassi, K; Lovenberg, TW; Carruthers, NI; Bhattacharya, A; Letavic, MA Identification of (R)-(2-Chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyridin-2-yl)-4-methyl-6,7-dihydro-1H-imidazo[4,5-c]pyridin-5(4H)-yl)methanone (JNJ 54166060), a Small Molecule Antagonist of the P2X7 receptor. J Med Chem59:8535-48 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM254160 |
|---|
| n/a |
|---|
| Name | BDBM254160 |
|---|
| Synonyms: | US10112937, Example 40 | US10150765, Example 40 | US10703749, Example 11 | US9464084, 40 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H15ClF4N4O |
|---|
| Mol. Mass. | 438.806 |
|---|
| SMILES | C[C@H]1N(CCc2c1ncn2-c1ccc(F)cn1)C(=O)c1cccc(c1Cl)C(F)(F)F |r| |
|---|
| Structure | 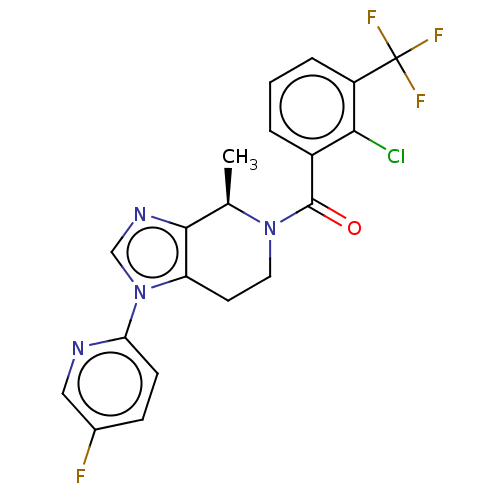
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Swanson, DM; Savall, BM; Coe, KJ; Schoetens, F; Koudriakova, T; Skaptason, J; Wall, J; Rech, J; Deng, X; De Angelis, M; Everson, A; Lord, B; Wang, Q; Ao, H; Scott, B; Sepassi, K; Lovenberg, TW; Carruthers, NI; Bhattacharya, A; Letavic, MA Identification of (R)-(2-Chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyridin-2-yl)-4-methyl-6,7-dihydro-1H-imidazo[4,5-c]pyridin-5(4H)-yl)methanone (JNJ 54166060), a Small Molecule Antagonist of the P2X7 receptor. J Med Chem59:8535-48 (2016) [PubMed] Article
Swanson, DM; Savall, BM; Coe, KJ; Schoetens, F; Koudriakova, T; Skaptason, J; Wall, J; Rech, J; Deng, X; De Angelis, M; Everson, A; Lord, B; Wang, Q; Ao, H; Scott, B; Sepassi, K; Lovenberg, TW; Carruthers, NI; Bhattacharya, A; Letavic, MA Identification of (R)-(2-Chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyridin-2-yl)-4-methyl-6,7-dihydro-1H-imidazo[4,5-c]pyridin-5(4H)-yl)methanone (JNJ 54166060), a Small Molecule Antagonist of the P2X7 receptor. J Med Chem59:8535-48 (2016) [PubMed] Article