

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343025 ((R)-4-(3-(methylamino)pyrrolidin-1-yl)-N2-neopenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356873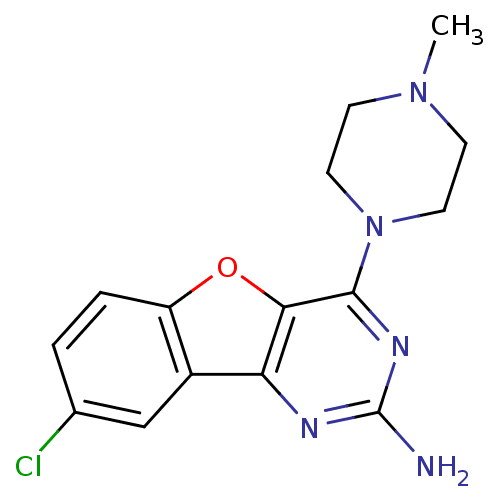 (CHEMBL1914541) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343021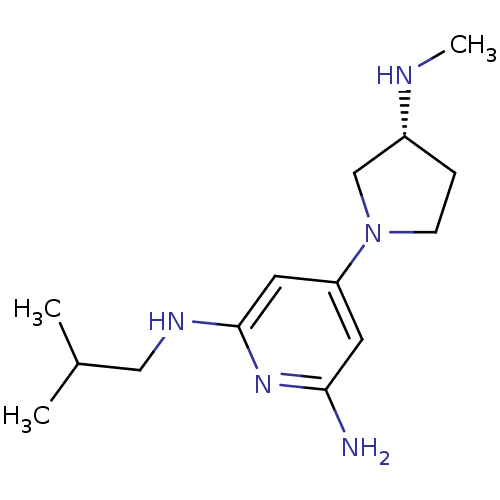 ((R)-N2-isobutyl-4-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319300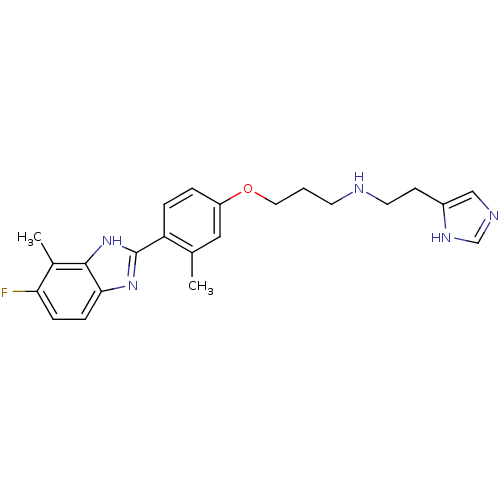 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343018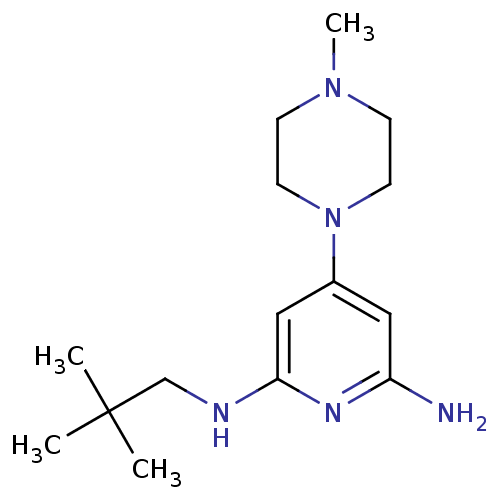 (4-(4-methylpiperazin-1-yl)-N2-neopentylpyridine-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356794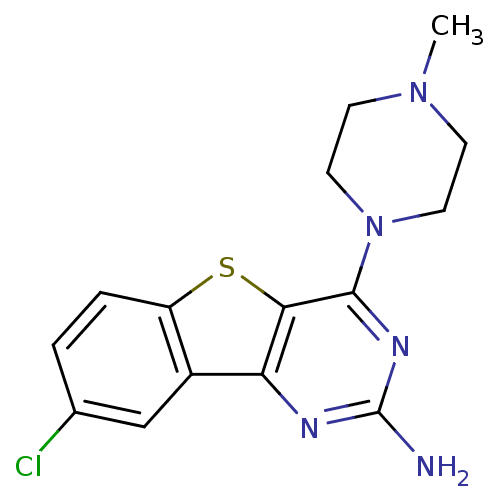 (CHEMBL1914462) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM160091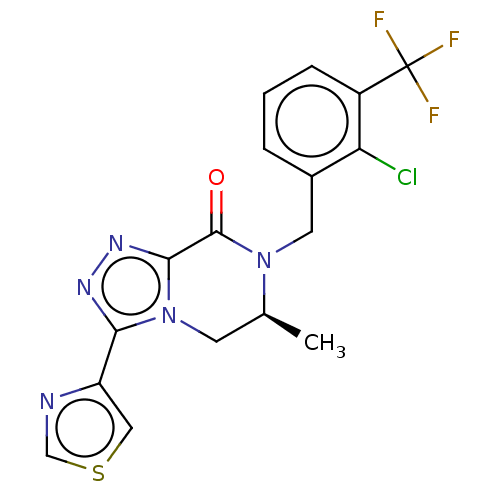 (US10047092, 32 | US10053463, 32 | US9040534, 32) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation... | US Patent US9040534 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM160091 (US10047092, 32 | US10053463, 32 | US9040534, 32) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ... | US Patent US10053463 (2018) BindingDB Entry DOI: 10.7270/Q2FT8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM48901 (BDBM160086 | US10047092, 27 | US10053463, 27 | US9...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation... | US Patent US9040534 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348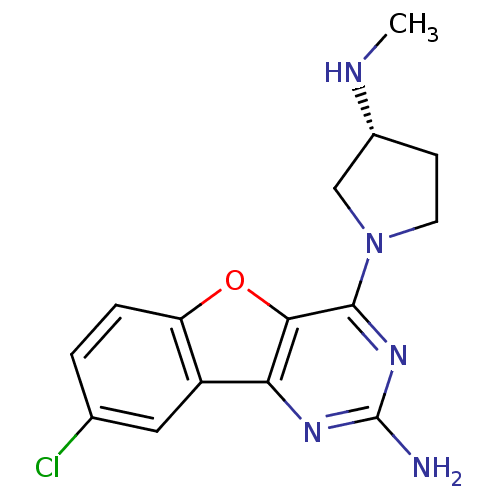 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM48901 (BDBM160086 | US10047092, 27 | US10053463, 27 | US9...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ... | US Patent US10053463 (2018) BindingDB Entry DOI: 10.7270/Q2FT8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM48901 (BDBM160086 | US10047092, 27 | US10053463, 27 | US9...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10047092 (2018) BindingDB Entry DOI: 10.7270/Q27D2X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM160091 (US10047092, 32 | US10053463, 32 | US9040534, 32) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10047092 (2018) BindingDB Entry DOI: 10.7270/Q27D2X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335426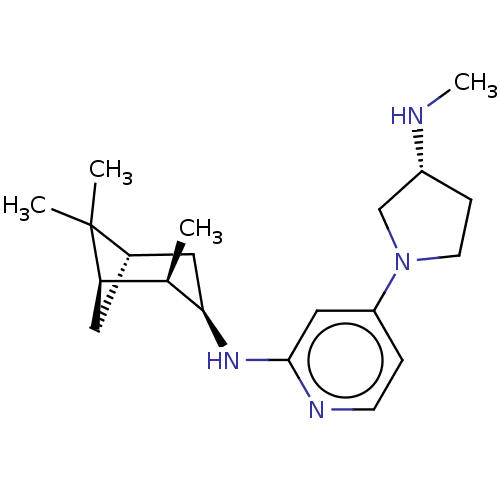 (4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1R,2R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356815 (CHEMBL1914781) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356788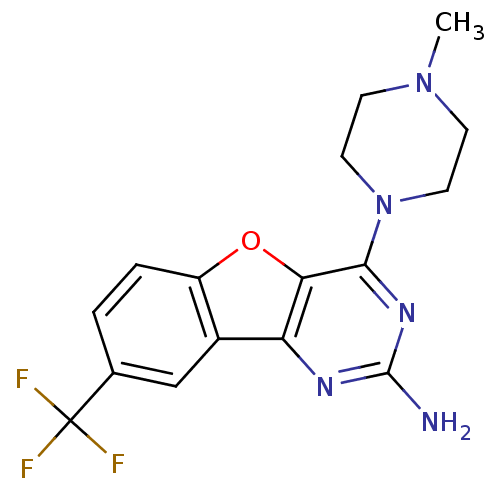 (CHEMBL1914755) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061017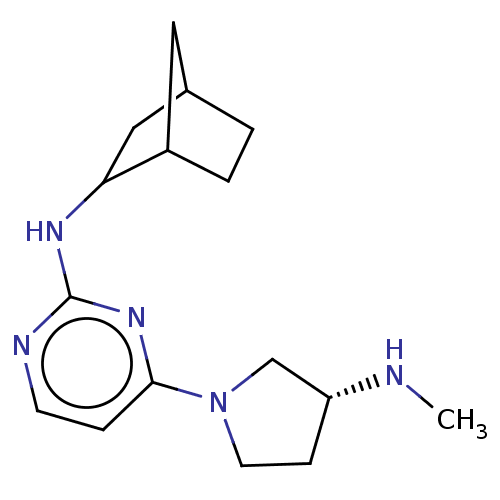 (CHEMBL3393547) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 25: 956-9 (2015) Article DOI: 10.1016/j.bmcl.2014.12.027 BindingDB Entry DOI: 10.7270/Q2639RDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343023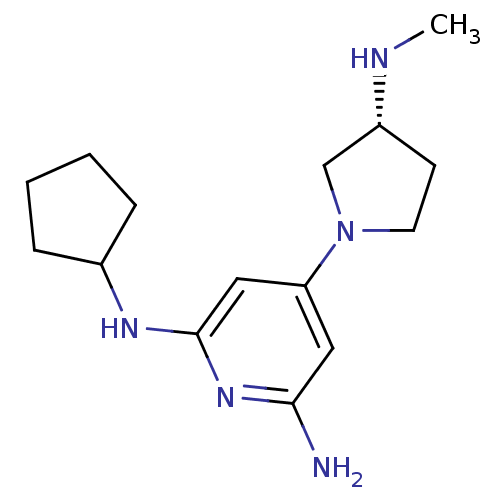 ((R)-N2-cyclopentyl-4-(3-(methylamino)pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006745 (CHEMBL3236556) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343007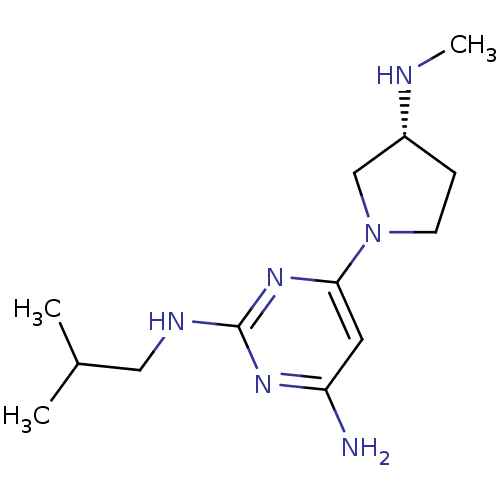 ((R)-N2-isobutyl-6-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM160085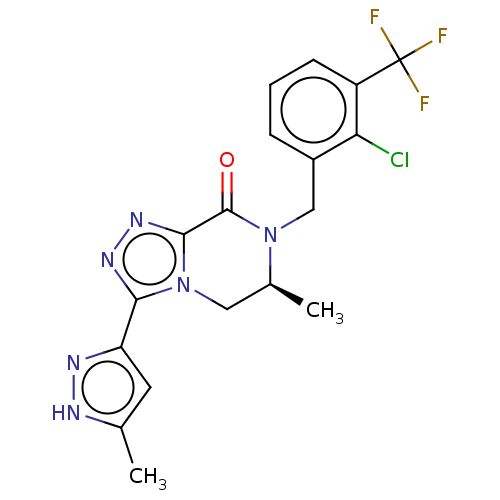 (US10047092, 26 | US10053463, 26 | US9040534, 26) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ... | US Patent US10053463 (2018) BindingDB Entry DOI: 10.7270/Q2FT8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061008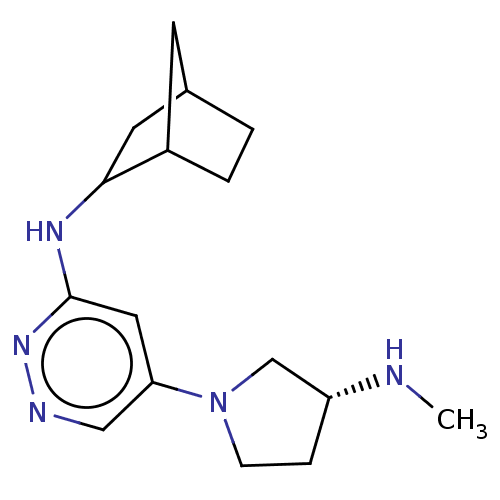 (CHEMBL3393556 | US9732087, 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061098 (CHEMBL3393534 | US9732087, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 25: 956-9 (2015) Article DOI: 10.1016/j.bmcl.2014.12.027 BindingDB Entry DOI: 10.7270/Q2639RDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006742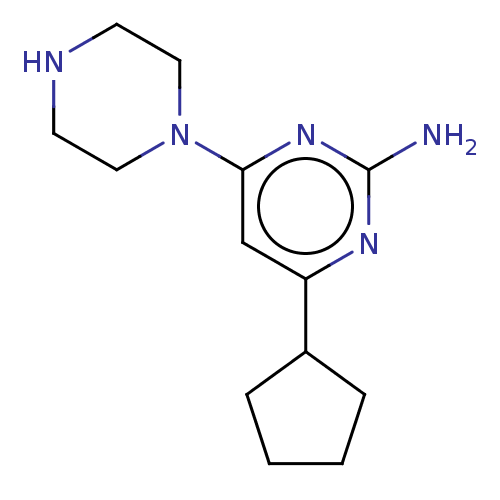 (CHEMBL3236553) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 24: 5489-92 (2015) Article DOI: 10.1016/j.bmcl.2014.10.013 BindingDB Entry DOI: 10.7270/Q25M67B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM160085 (US10047092, 26 | US10053463, 26 | US9040534, 26) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation... | US Patent US9040534 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342990 (CHEMBL1771001 | N4-cyclopentyl-6-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356817 (CHEMBL1914783) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356793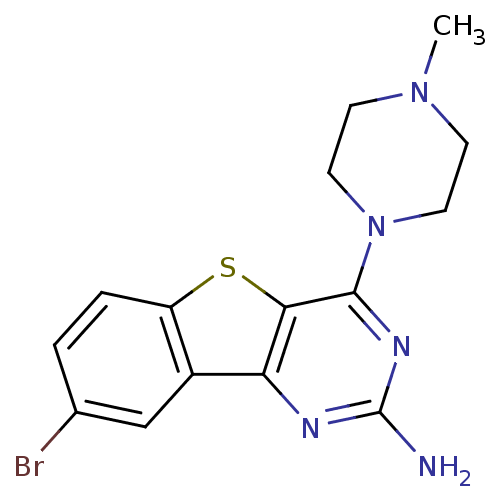 (CHEMBL1914760) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335425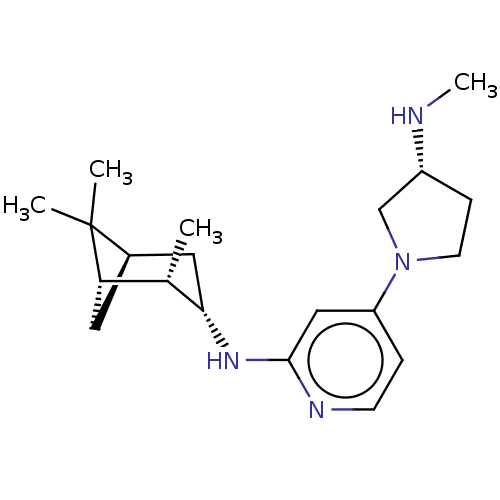 (4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061098 (CHEMBL3393534 | US9732087, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320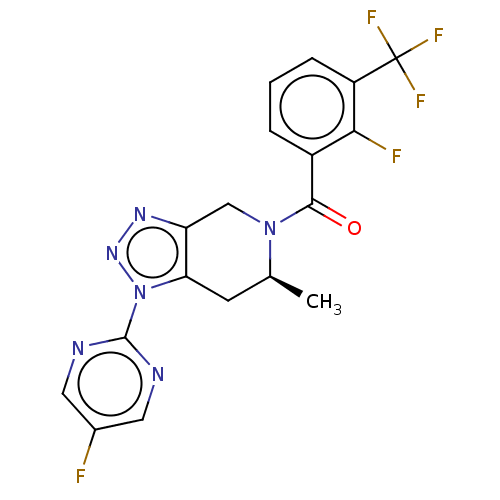 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM160085 (US10047092, 26 | US10053463, 26 | US9040534, 26) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10047092 (2018) BindingDB Entry DOI: 10.7270/Q27D2X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006743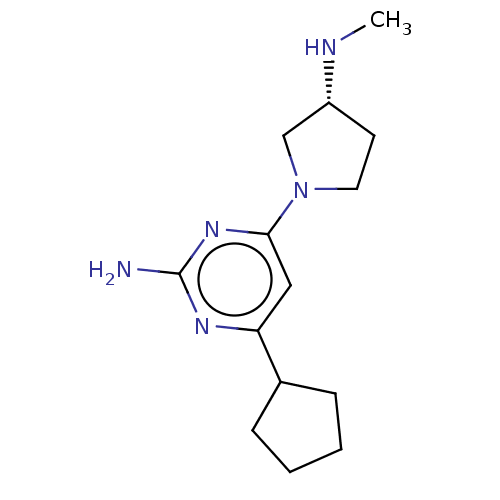 (CHEMBL3236554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM246865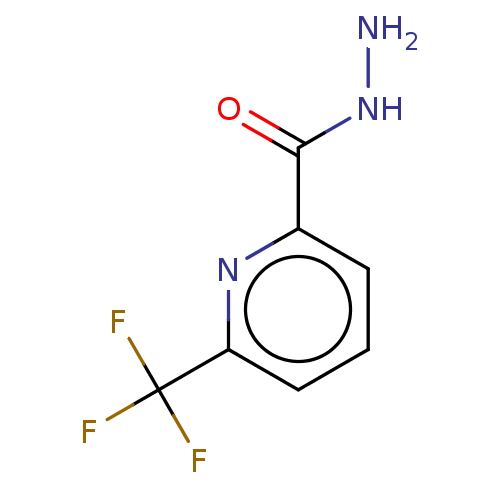 (6-(trifluoromethyl)picolinohydrazide | US10053463,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ... | US Patent US10053463 (2018) BindingDB Entry DOI: 10.7270/Q2FT8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356874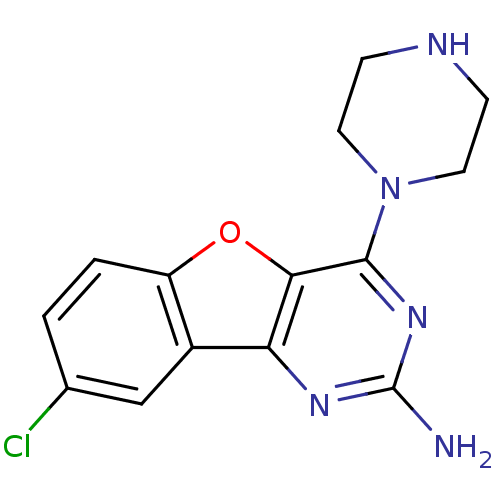 (CHEMBL1914542) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356872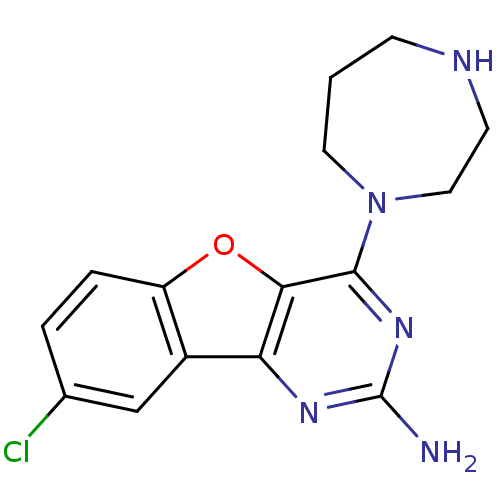 (CHEMBL1914540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor | Bioorg Med Chem Lett 21: 6577-81 (2011) Article DOI: 10.1016/j.bmcl.2011.08.014 BindingDB Entry DOI: 10.7270/Q2CN749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM160075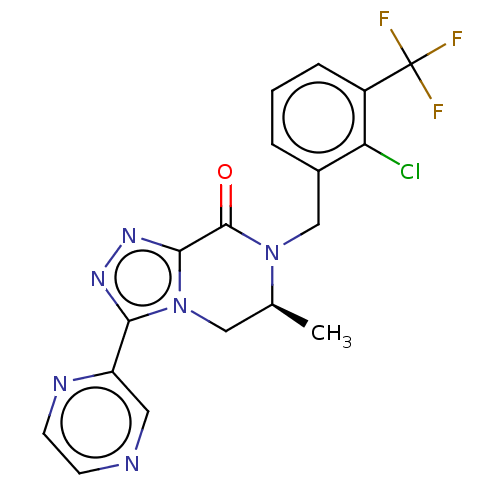 (US10047092, 16 | US9040534, 16) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10047092 (2018) BindingDB Entry DOI: 10.7270/Q27D2X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM160075 (US10047092, 16 | US9040534, 16) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation... | US Patent US9040534 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10703749 (2020) BindingDB Entry DOI: 10.7270/Q2JD50VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US9464084 (2016) BindingDB Entry DOI: 10.7270/Q27D2T3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006742 (CHEMBL3236553) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10150765 (2018) BindingDB Entry DOI: 10.7270/Q23T9K8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM227806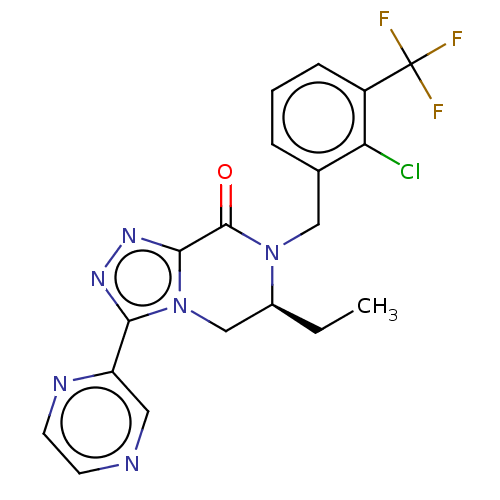 ((6S)-7-[2-Chloro-3-(trifluoromethyl)benzyl]-6-ethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ... | US Patent US10053463 (2018) BindingDB Entry DOI: 10.7270/Q2FT8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM227806 ((6S)-7-[2-Chloro-3-(trifluoromethyl)benzyl]-6-ethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ... | US Patent US10053463 (2018) BindingDB Entry DOI: 10.7270/Q2FT8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM160130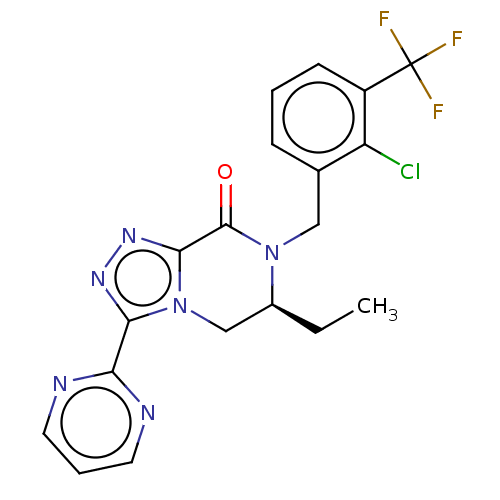 (US10047092, 72 | US10053463, 72 | US9040534, 71 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation... | US Patent US9040534 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM227806 ((6S)-7-[2-Chloro-3-(trifluoromethyl)benzyl]-6-ethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10047092 (2018) BindingDB Entry DOI: 10.7270/Q27D2X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM160080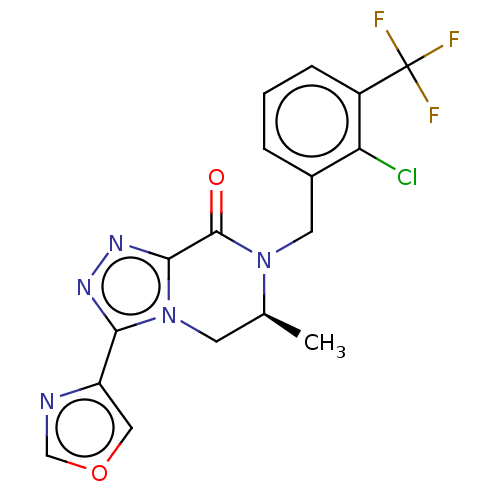 (US10047092, 21 | US10053463, 21 | US9040534, 21) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according... | US Patent US10047092 (2018) BindingDB Entry DOI: 10.7270/Q27D2X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006739 (CHEMBL3236552) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11547 total ) | Next | Last >> |