| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Protein mono-ADP-ribosyltransferase PARP14 |
|---|
| Ligand | BDBM434484 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Enzymatic Assay |
|---|
| IC50 | <1000±n/a nM |
|---|
| Citation |  Schenkel, LB; Vasbinder, MM; Kuntz, KW; Swinger, KK Quinazolinones as PARP14 inhibitors US Patent US11008308 Publication Date 5/18/2021 Schenkel, LB; Vasbinder, MM; Kuntz, KW; Swinger, KK Quinazolinones as PARP14 inhibitors US Patent US11008308 Publication Date 5/18/2021 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Protein mono-ADP-ribosyltransferase PARP14 |
|---|
| Name: | Protein mono-ADP-ribosyltransferase PARP14 |
|---|
| Synonyms: | (ARTD8 or PARP14) | (ARTD8 or PARP14, Y1660L) | 2.4.2.- | 2.4.2.30 | ADP-ribosyltransferase diphtheria toxin-like 8 | ARTD8 | B aggressive lymphoma protein 2 | BAL2 | BAL2 | Human diphtheria toxin-like ADP-ribosyltransferase (ARTD8 or PARP14, Y1660L) | KIAA1268 | KIAA1268 GN | KIAA1268 GN | PAR14_HUMAN | PARP-14 | PARP14 | Poly [ADP-ribose] polymerase 14 | Synonyms=BAL2 |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 202812.41 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q460N5 |
|---|
| Residue: | 1801 |
|---|
| Sequence: | MAVPGSFPLLVEGSWGPDPPKNLNTKLQMYFQSPKRSGGGECEVRQDPRSPSRFLVFFYP
EDVRQKVLERKNHELVWQGKGTFKLTVQLPATPDEIDHVFEEELLTKESKTKEDVKEPDV
SEELDTKLPLDGGLDKMEDIPEECENISSLVAFENLKANVTDIMLILLVENISGLSNDDF
QVEIIRDFDVAVVTFQKHIDTIRFVDDCTKHHSIKQLQLSPRLLEVTNTIRVENLPPGAD
DYSLKLFFENPYNGGGRVANVEYFPEESSALIEFFDRKVLDTIMATKLDFNKMPLSVFPY
YASLGTALYGKEKPLIKLPAPFEESLDLPLWKFLQKKNHLIEEINDEMRRCHCELTWSQL
SGKVTIRPAATLVNEGRPRIKTWQADTSTTLSSIRSKYKVNPIKVDPTMWDTIKNDVKDD
RILIEFDTLKEMVILAGKSEDVQSIEVQVRELIESTTQKIKREEQSLKEKMIISPGRYFL
LCHSSLLDHLLTECPEIEICYDRVTQHLCLKGPSADVYKAKCEIQEKVYTMAQKNIQVSP
EIFQFLQQVNWKEFSKCLFIAQKILALYELEGTTVLLTSCSSEALLEAEKQMLSALNYKR
IEVENKEVLHGKKWKGLTHNLLKKQNSSPNTVIINELTSETTAEVIITGCVKEVNETYKL
LFNFVEQNMKIERLVEVKPSLVIDYLKTEKKLFWPKIKKVNVQVSFNPENKQKGILLTGS
KTEVLKAVDIVKQVWDSVCVKSVHTDKPGAKQFFQDKARFYQSEIKRLFGCYIELQENEV
MKEGGSPAGQKCFSRTVLAPGVVLIVQQGDLARLPVDVVVNASNEDLKHYGGLAAALSKA
AGPELQADCDQIVKREGRLLPGNATISKAGKLPYHHVIHAVGPRWSGYEAPRCVYLLRRA
VQLSLCLAEKYKYRSIAIPAISSGVFGFPLGRCVETIVSAIKENFQFKKDGHCLKEIYLV
DVSEKTVEAFAEAVKTVFKATLPDTAAPPGLPPAAAGPGKTSWEKGSLVSPGGLQMLLVK
EGVQNAKTDVVVNSVPLDLVLSRGPLSKSLLEKAGPELQEELDTVGQGVAVSMGTVLKTS
SWNLDCRYVLHVVAPEWRNGSTSSLKIMEDIIRECMEITESLSLKSIAFPAIGTGNLGFP
KNIFAELIISEVFKFSSKNQLKTLQEVHFLLHPSDHENIQAFSDEFARRANGNLVSDKIP
KAKDTQGFYGTVSSPDSGVYEMKIGSIIFQVASGDITKEEADVIVNSTSNSFNLKAGVSK
AILECAGQNVERECSQQAQQRKNDYIITGGGFLRCKNIIHVIGGNDVKSSVSSVLQECEK
KNYSSICLPAIGTGNAKQHPDKVAEAIIDAIEDFVQKGSAQSVKKVKVVIFLPQVLDVFY
ANMKKREGTQLSSQQSVMSKLASFLGFSKQSPQKKNHLVLEKKTESATFRVCGENVTCVE
YAISWLQDLIEKEQCPYTSEDECIKDFDEKEYQELNELQKKLNINISLDHKRPLIKVLGI
SRDVMQARDEIEAMIKRVRLAKEQESRADCISEFIEWQYNDNNTSHCFNKMTNLKLEDAR
REKKKTVDVKINHRHYTVNLNTYTATDTKGHSLSVQRLTKSKVDIPAHWSDMKQQNFCVV
ELLPSDPEYNTVASKFNQTCSHFRIEKIERIQNPDLWNSYQAKKKTMDAKNGQTMNEKQL
FHGTDAGSVPHVNRNGFNRSYAGKNAVAYGKGTYFAVNANYSANDTYSRPDANGRKHVYY
VRVLTGIYTHGNHSLIVPPSKNPQNPTDLYDTVTDNVHHPSLFVAFYDYQAYPEYLITFR
K
|
|
|
|---|
| BDBM434484 |
|---|
| n/a |
|---|
| Name | BDBM434484 |
|---|
| Synonyms: | US10562891, Example 398 | US11008308, Example 398 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H30FN3O4S |
|---|
| Mol. Mass. | 463.565 |
|---|
| SMILES | CCC(=O)N1CCC(COc2cc(F)c3c(c2)nc(CSC2CCOCC2)[nH]c3=O)CC1 |
|---|
| Structure | 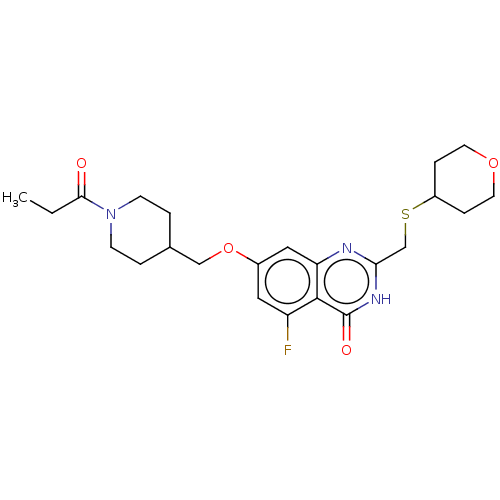
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Schenkel, LB; Vasbinder, MM; Kuntz, KW; Swinger, KK Quinazolinones as PARP14 inhibitors US Patent US11008308 Publication Date 5/18/2021
Schenkel, LB; Vasbinder, MM; Kuntz, KW; Swinger, KK Quinazolinones as PARP14 inhibitors US Patent US11008308 Publication Date 5/18/2021