 Found 4504 hits with Last Name = 'schenkel' and Initial = 'lb'
Found 4504 hits with Last Name = 'schenkel' and Initial = 'lb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM154061
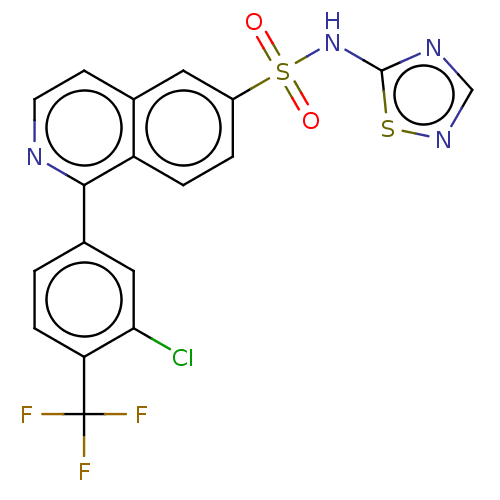
(US9012443, 57)Show SMILES FC(F)(F)c1ccc(cc1Cl)-c1nccc2cc(ccc12)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C18H10ClF3N4O2S2/c19-15-8-11(1-4-14(15)18(20,21)22)16-13-3-2-12(7-10(13)5-6-23-16)30(27,28)26-17-24-9-25-29-17/h1-9H,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533552
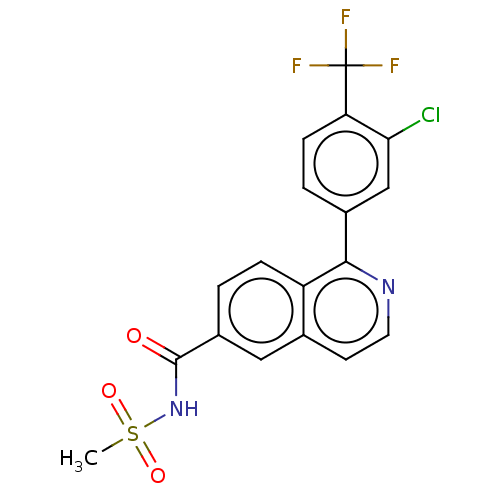
(CHEMBL4450471)Show SMILES CS(=O)(=O)NC(=O)c1ccc2c(nccc2c1)-c1ccc(c(Cl)c1)C(F)(F)F Show InChI InChI=1S/C18H12ClF3N2O3S/c1-28(26,27)24-17(25)12-2-4-13-10(8-12)6-7-23-16(13)11-3-5-14(15(19)9-11)18(20,21)22/h2-9H,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533547

(CHEMBL4537339)Show SMILES CS(=O)(=O)NC(=O)c1cc(F)c(Oc2ccc(Cl)c(Cl)c2)cc1F Show InChI InChI=1S/C14H9Cl2F2NO4S/c1-24(21,22)19-14(20)8-5-12(18)13(6-11(8)17)23-7-2-3-9(15)10(16)4-7/h2-6H,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50272533
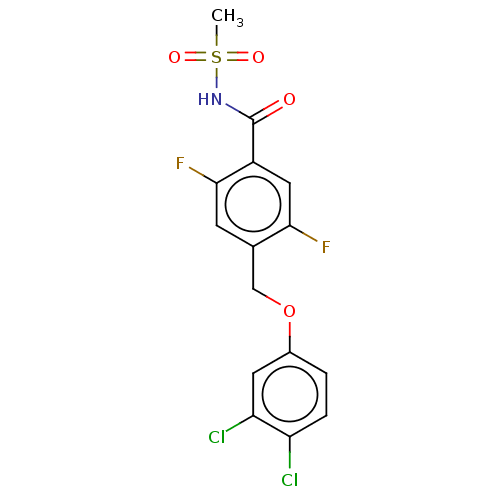
(CHEMBL4129030)Show SMILES CS(=O)(=O)NC(=O)c1cc(F)c(COc2ccc(Cl)c(Cl)c2)cc1F Show InChI InChI=1S/C15H11Cl2F2NO4S/c1-25(22,23)20-15(21)10-6-13(18)8(4-14(10)19)7-24-9-2-3-11(16)12(17)5-9/h2-6H,7H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533554

(CHEMBL4470763)Show SMILES CC(C)COc1ncc(cc1Cl)-c1cc(F)c(cc1F)C(=O)NS(C)(=O)=O Show InChI InChI=1S/C17H17ClF2N2O4S/c1-9(2)8-26-17-13(18)4-10(7-21-17)11-5-15(20)12(6-14(11)19)16(23)22-27(3,24)25/h4-7,9H,8H2,1-3H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533553

(CHEMBL4445237)Show SMILES CS(=O)(=O)NC(=O)c1cc(F)c(cc1F)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H9Cl2F2NO3S/c1-23(21,22)19-14(20)9-6-12(17)8(5-13(9)18)7-2-3-10(15)11(16)4-7/h2-6H,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM329203
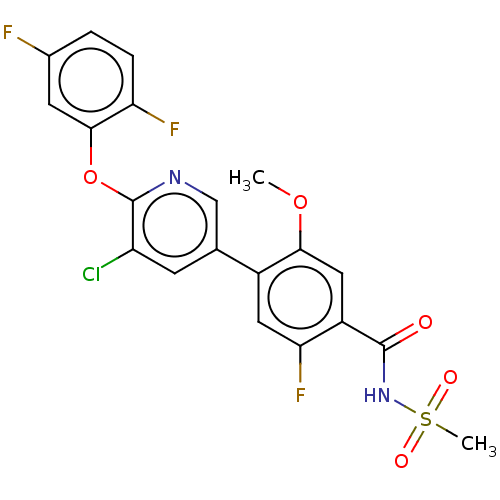
(4-(5-Chloro-6-((1-Methylcyclopropyl)Methoxy)Pyridi...)Show SMILES COc1cc(C(=O)NS(C)(=O)=O)c(F)cc1-c1cnc(Oc2cc(F)ccc2F)c(Cl)c1 Show InChI InChI=1S/C20H14ClF3N2O5S/c1-30-17-8-13(19(27)26-32(2,28)29)16(24)7-12(17)10-5-14(21)20(25-9-10)31-18-6-11(22)3-4-15(18)23/h3-9H,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533549
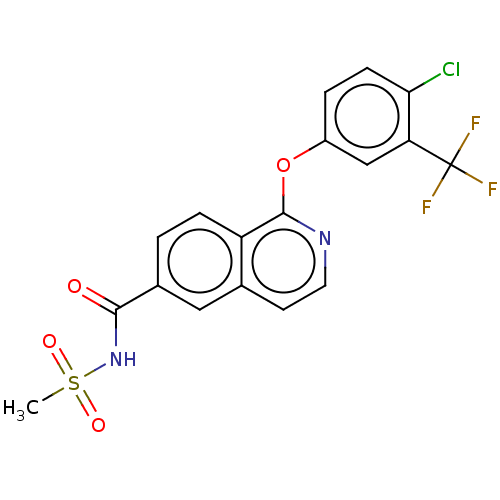
(CHEMBL4579742)Show SMILES CS(=O)(=O)NC(=O)c1ccc2c(Oc3ccc(Cl)c(c3)C(F)(F)F)nccc2c1 Show InChI InChI=1S/C18H12ClF3N2O4S/c1-29(26,27)24-16(25)11-2-4-13-10(8-11)6-7-23-17(13)28-12-3-5-15(19)14(9-12)18(20,21)22/h2-9H,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533546

(CHEMBL4462738)Show SMILES CC(C)COc1ncc(Oc2nccc3cc(ccc23)C(=O)NS(C)(=O)=O)cc1Cl Show InChI InChI=1S/C20H20ClN3O5S/c1-12(2)11-28-20-17(21)9-15(10-23-20)29-19-16-5-4-14(8-13(16)6-7-22-19)18(25)24-30(3,26)27/h4-10,12H,11H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50269118

(CHEMBL4100413)Show SMILES FC(F)Oc1cccnc1-c1cccc(c1)C(=O)Nc1cccc(c1-c1cccnc1)C(F)(F)F Show InChI InChI=1S/C25H16F5N3O2/c26-24(27)35-20-10-4-12-32-22(20)15-5-1-6-16(13-15)23(34)33-19-9-2-8-18(25(28,29)30)21(19)17-7-3-11-31-14-17/h1-14,24H,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 3817-3824 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.054
BindingDB Entry DOI: 10.7270/Q2RB773S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533541
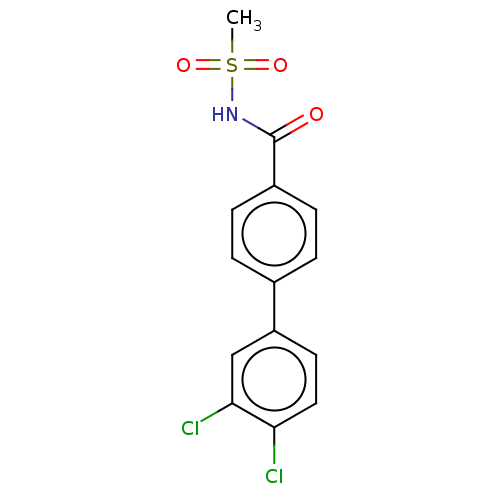
(CHEMBL4559824)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H11Cl2NO3S/c1-21(19,20)17-14(18)10-4-2-9(3-5-10)11-6-7-12(15)13(16)8-11/h2-8H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK3 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361821

(CHEMBL1938654)Show SMILES CNC(=O)c1cnc(N)c2cc(sc12)-c1ccc(cc1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C19H22N4O3S2/c1-19(2,3)23-28(25,26)12-7-5-11(6-8-12)15-9-13-16(27-15)14(18(24)21-4)10-22-17(13)20/h5-10,23H,1-4H3,(H2,20,22)(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341035

(1-(4-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-b...)Show SMILES CNC(=O)Nc1ccc(Nc2nc3ccccc3n2-c2nc(C)nc(N)n2)cc1 Show InChI InChI=1S/C19H19N9O/c1-11-22-16(20)27-17(23-11)28-15-6-4-3-5-14(15)26-18(28)24-12-7-9-13(10-8-12)25-19(29)21-2/h3-10H,1-2H3,(H,24,26)(H2,21,25,29)(H2,20,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361826

(CHEMBL1938659)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(cc1)-c1cc2c(N)ncc(C(=O)NCCN3CCOCC3)c2s1 Show InChI InChI=1S/C24H31N5O4S2/c1-24(2,3)28-35(31,32)17-6-4-16(5-7-17)20-14-18-21(34-20)19(15-27-22(18)25)23(30)26-8-9-29-10-12-33-13-11-29/h4-7,14-15,28H,8-13H2,1-3H3,(H2,25,27)(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361823
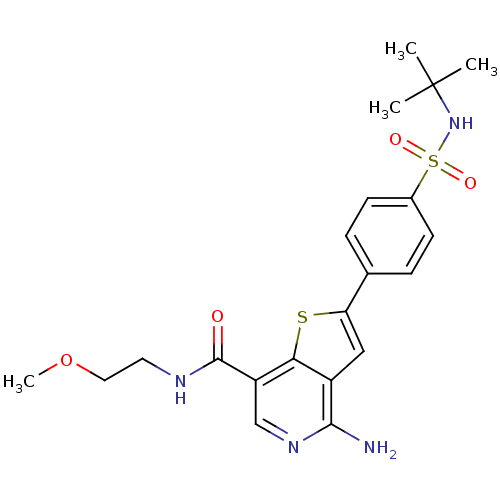
(CHEMBL1938656)Show SMILES COCCNC(=O)c1cnc(N)c2cc(sc12)-c1ccc(cc1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C21H26N4O4S2/c1-21(2,3)25-31(27,28)14-7-5-13(6-8-14)17-11-15-18(30-17)16(12-24-19(15)22)20(26)23-9-10-29-4/h5-8,11-12,25H,9-10H2,1-4H3,(H2,22,24)(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341054
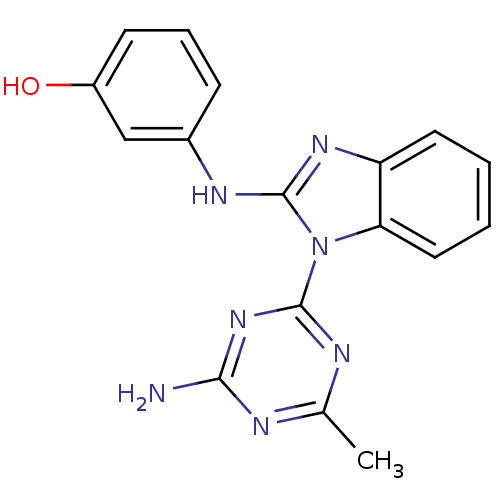
(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK1 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361820

(CHEMBL1938653)Show SMILES CNC(=O)c1cnc(N)c2cc(sc12)-c1ccc(cc1)C(C)(C)C#N Show InChI InChI=1S/C19H18N4OS/c1-19(2,10-20)12-6-4-11(5-7-12)15-8-13-16(25-15)14(18(24)22-3)9-23-17(13)21/h4-9H,1-3H3,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361825
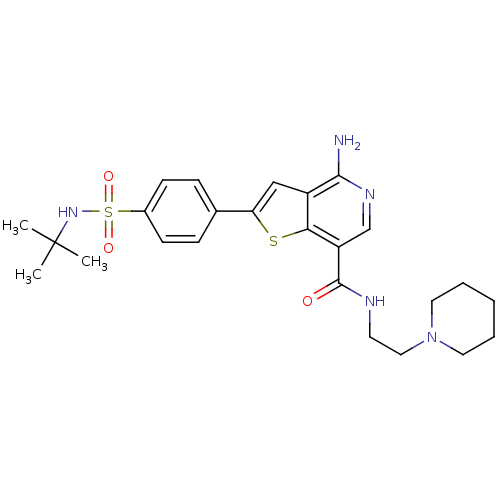
(CHEMBL1938658)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(cc1)-c1cc2c(N)ncc(C(=O)NCCN3CCCCC3)c2s1 Show InChI InChI=1S/C25H33N5O3S2/c1-25(2,3)29-35(32,33)18-9-7-17(8-10-18)21-15-19-22(34-21)20(16-28-23(19)26)24(31)27-11-14-30-12-5-4-6-13-30/h7-10,15-16,29H,4-6,11-14H2,1-3H3,(H2,26,28)(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361827

(CHEMBL1938660)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(cc1)-c1cc2c(N)ncc(C(=O)NCCCN3CCOCC3)c2s1 Show InChI InChI=1S/C25H33N5O4S2/c1-25(2,3)29-36(32,33)18-7-5-17(6-8-18)21-15-19-22(35-21)20(16-28-23(19)26)24(31)27-9-4-10-30-11-13-34-14-12-30/h5-8,15-16,29H,4,9-14H2,1-3H3,(H2,26,28)(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217696
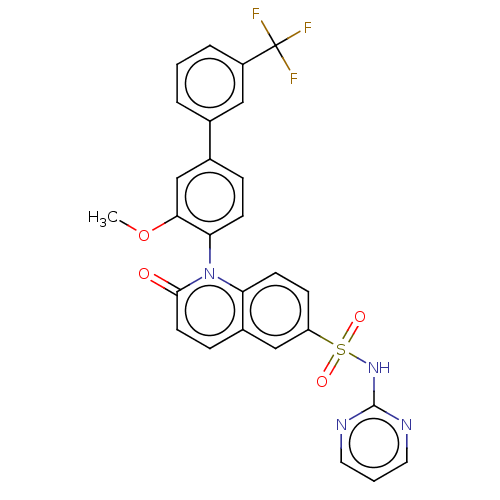
(US9212182, 672)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C27H19F3N4O4S/c1-38-24-16-18(17-4-2-5-20(14-17)27(28,29)30)6-9-23(24)34-22-10-8-21(15-19(22)7-11-25(34)35)39(36,37)33-26-31-12-3-13-32-26/h2-16H,1H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of slow inactivated human NaV1.7 expressed in HEK293 cells at -20 mV holding potential after 5 mins by IonWorks electrophysiology method |
Bioorg Med Chem Lett 27: 3817-3824 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.054
BindingDB Entry DOI: 10.7270/Q2RB773S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human U-87 cells assessed as inhibition of phosphorylation of AKT at S473 |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341035

(1-(4-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-b...)Show SMILES CNC(=O)Nc1ccc(Nc2nc3ccccc3n2-c2nc(C)nc(N)n2)cc1 Show InChI InChI=1S/C19H19N9O/c1-11-22-16(20)27-17(23-11)28-15-6-4-3-5-14(15)26-18(28)24-12-7-9-13(10-8-12)25-19(29)21-2/h3-10H,1-2H3,(H,24,26)(H2,21,25,29)(H2,20,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50269128

(CHEMBL4079509)Show SMILES FC(F)Oc1cccnc1-c1cccc(c1)C(=O)Nc1cccc(c1-c1ccccn1)C(F)(F)F Show InChI InChI=1S/C25H16F5N3O2/c26-24(27)35-20-11-5-13-32-22(20)15-6-3-7-16(14-15)23(34)33-19-10-4-8-17(25(28,29)30)21(19)18-9-1-2-12-31-18/h1-14,24H,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of slow inactivated human NaV1.7 expressed in HEK293 cells at -20 mV holding potential after 5 mins by IonWorks electrophysiology method |
Bioorg Med Chem Lett 27: 3817-3824 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.054
BindingDB Entry DOI: 10.7270/Q2RB773S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361822

(CHEMBL1938655)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(cc1)-c1cc2c(N)ncc(C(=O)NCCO)c2s1 Show InChI InChI=1S/C20H24N4O4S2/c1-20(2,3)24-30(27,28)13-6-4-12(5-7-13)16-10-14-17(29-16)15(11-23-18(14)21)19(26)22-8-9-25/h4-7,10-11,24-25H,8-9H2,1-3H3,(H2,21,23)(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361824

(CHEMBL1938657)Show SMILES CN(C)CCNC(=O)c1cnc(N)c2cc(sc12)-c1ccc(cc1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C22H29N5O3S2/c1-22(2,3)26-32(29,30)15-8-6-14(7-9-15)18-12-16-19(31-18)17(13-25-20(16)23)21(28)24-10-11-27(4)5/h6-9,12-13,26H,10-11H2,1-5H3,(H2,23,25)(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... |
J Med Chem 54: 8440-50 (2011)
Article DOI: 10.1021/jm200911r
BindingDB Entry DOI: 10.7270/Q22N52PG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341063
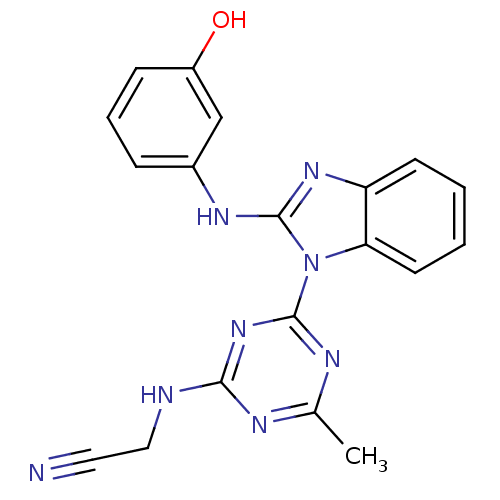
(2-(4-(2-(3-hydroxyphenylamino)-1H-benzo[d]imidazol...)Show SMILES Cc1nc(NCC#N)nc(n1)-n1c(Nc2cccc(O)c2)nc2ccccc12 Show InChI InChI=1S/C19H16N8O/c1-12-22-17(21-10-9-20)26-18(23-12)27-16-8-3-2-7-15(16)25-19(27)24-13-5-4-6-14(28)11-13/h2-8,11,28H,10H2,1H3,(H,24,25)(H,21,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM50269118

(CHEMBL4100413)Show SMILES FC(F)Oc1cccnc1-c1cccc(c1)C(=O)Nc1cccc(c1-c1cccnc1)C(F)(F)F Show InChI InChI=1S/C25H16F5N3O2/c26-24(27)35-20-10-4-12-32-22(20)15-5-1-6-16(13-15)23(34)33-19-9-2-8-18(25(28,29)30)21(19)17-7-3-11-31-14-17/h1-14,24H,(H,33,34) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of slow inactivated mouse NaV1.7 expressed in HEK293 cells at -20 mV holding potential after 5 mins by IonWorks electrophysiology method |
Bioorg Med Chem Lett 27: 3817-3824 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.054
BindingDB Entry DOI: 10.7270/Q2RB773S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50326206
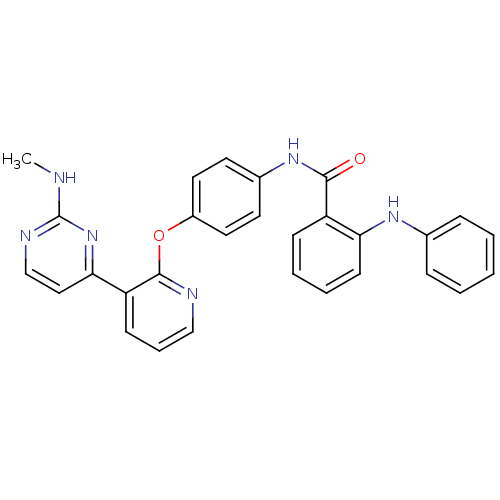
(CHEMBL1243200 | N-(4-(3-(2-(Methylamino)pyrimidin-...)Show SMILES CNc1nccc(n1)-c1cccnc1Oc1ccc(NC(=O)c2ccccc2Nc2ccccc2)cc1 Show InChI InChI=1S/C29H24N6O2/c1-30-29-32-19-17-26(35-29)24-11-7-18-31-28(24)37-22-15-13-21(14-16-22)34-27(36)23-10-5-6-12-25(23)33-20-8-3-2-4-9-20/h2-19,33H,1H3,(H,34,36)(H,30,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B by HTRF assay |
J Med Chem 53: 6368-77 (2010)
Article DOI: 10.1021/jm100394y
BindingDB Entry DOI: 10.7270/Q2C829H0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human U-87 cells assessed as inhibition of phosphorylation of AKT at S473 |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587933

(US11535621, Example 183)Show SMILES CO[C@H]1CC[C@@H](CC1)NC(=O)c1nc(nc2cn[nH]c12)-n1ccnc1 |r,wU:5.8,wD:2.1,(6.6,5.48,;6.6,3.94,;5.27,3.17,;3.89,3.91,;2.55,3.14,;2.55,1.6,;3.89,.83,;5.22,1.6,;1.22,.83,;1.22,-.71,;2.55,-1.48,;-.11,-1.48,;-1.45,-.71,;-2.78,-1.48,;-2.78,-3.02,;-1.45,-3.79,;-1.13,-5.29,;.4,-5.45,;1.03,-4.05,;-.11,-3.02,;-4.12,-.71,;-5.52,-1.33,;-6.55,-.19,;-5.78,1.15,;-4.28,.83,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587934

(US11535621, Example 184)Show SMILES Cc1cc2nc(nc(C(=O)N[C@H]3CC[C@@H](CC3)C(C)(C)O)c2[nH]1)-n1ccnc1 |r,wU:11.10,wD:14.17,(.51,-6.02,;-.26,-4.68,;-1.8,-4.52,;-2.12,-3.02,;-3.45,-2.25,;-3.45,-.71,;-2.12,.06,;-.78,-.71,;.55,.06,;1.89,-.71,;.55,1.6,;1.89,2.37,;1.89,3.91,;3.22,4.68,;4.55,3.91,;4.55,2.37,;3.22,1.6,;5.89,4.68,;5.12,6.02,;6.66,6.02,;7.22,3.91,;-.78,-2.25,;.36,-3.28,;-4.78,.06,;-6.19,-.56,;-7.22,.58,;-6.45,1.92,;-4.94,1.6,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587935

(US11535621, Example 185)Show SMILES O=C(N[C@H]1CC[C@H](CC#N)CC1)c1nc(nc2cc[nH]c12)-n1ccnc1 |r,wU:3.2,wD:6.6,(1.22,-.71,;-.11,.06,;-.11,1.6,;1.22,2.37,;1.22,3.91,;2.55,4.68,;3.89,3.91,;5.22,4.68,;5.22,6.22,;5.22,7.76,;3.89,2.37,;2.55,1.6,;-1.45,-.71,;-2.78,.06,;-4.12,-.71,;-4.12,-2.25,;-2.78,-3.02,;-2.46,-4.52,;-.93,-4.68,;-.3,-3.28,;-1.45,-2.25,;-5.45,.06,;-6.86,-.56,;-7.89,.58,;-7.12,1.92,;-5.61,1.6,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587940

(US11535621, Example 190)Show SMILES CN(C)CC#Cc1ccc(NC(=O)c2nc(nc3cc[nH]c23)-n2ccnc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587942

(N-((1S,4r)-4-((S)-1-hydroxyethyl)cyclohexyl)-5-(1H...)Show SMILES C[C@H](O)[C@H]1CC[C@@H](CC1)NC(=O)c1nc(cc2cn[nH]c12)-n1ccnc1 |r,wU:3.2,wD:6.9,1.1,(5.89,13.2,;5.89,11.66,;7.22,10.89,;4.55,10.89,;4.55,9.35,;3.22,8.58,;1.89,9.35,;1.89,10.89,;3.22,11.66,;.55,8.58,;.55,7.04,;1.89,6.27,;-.78,6.27,;-2.12,7.04,;-3.45,6.27,;-3.45,4.73,;-2.12,3.96,;-1.8,2.46,;-.26,2.29,;.36,3.7,;-.78,4.73,;-4.78,7.04,;-6.19,6.42,;-7.22,7.56,;-6.45,8.89,;-4.94,8.57,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587943

(N-((1S,4r)-4-((S)-1-hydroxyethyl)cyclohexyl)-5-(1H...)Show SMILES C[C@@H](O)[C@H]1CC[C@@H](CC1)NC(=O)c1nc(cc2cn[nH]c12)-n1ccnc1 |r,wU:3.2,1.1,wD:6.9,(5.89,13.2,;5.89,11.66,;7.22,10.89,;4.55,10.89,;4.55,9.35,;3.22,8.58,;1.89,9.35,;1.89,10.89,;3.22,11.66,;.55,8.58,;.55,7.04,;1.89,6.27,;-.78,6.27,;-2.12,7.04,;-3.45,6.27,;-3.45,4.73,;-2.12,3.96,;-1.8,2.46,;-.26,2.29,;.36,3.7,;-.78,4.73,;-4.78,7.04,;-6.19,6.42,;-7.22,7.56,;-6.45,8.89,;-4.94,8.57,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587951

(US11535621, Example 200)Show SMILES OCC[C@H]1CC[C@@H](CC1)NC(=O)c1nc(nc2cc[nH]c12)-n1ccnc1 |r,wU:6.9,wD:3.2,(7.89,4.68,;6.55,3.91,;5.22,4.68,;3.89,3.91,;2.55,4.68,;1.22,3.91,;1.22,2.37,;2.55,1.6,;3.89,2.37,;-.11,1.6,;-.11,.06,;1.22,-.71,;-1.45,-.71,;-2.78,.06,;-4.12,-.71,;-4.12,-2.25,;-2.78,-3.02,;-2.46,-4.52,;-.93,-4.68,;-.3,-3.28,;-1.45,-2.25,;-5.45,.06,;-6.86,-.56,;-7.89,.58,;-7.12,1.92,;-5.61,1.6,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587956

(US11535621, Example 205)Show SMILES O=C(Nc1ccc(cc1)C#N)c1nc(cc2cn[nH]c12)-n1ccnc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM587961

(US11535621, Example 210)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1nc(nc2ccsc12)-n1ccnc1 |r,wU:7.10,wD:4.3,(5.89,5.45,;5.89,3.91,;7.22,4.68,;7.22,3.14,;4.55,3.14,;3.22,3.91,;1.89,3.14,;1.89,1.6,;3.22,.83,;4.55,1.6,;.55,.83,;.55,-.71,;1.89,-1.48,;-.78,-1.48,;-2.12,-.71,;-3.45,-1.48,;-3.45,-3.02,;-2.12,-3.79,;-1.8,-5.29,;-.26,-5.45,;.36,-4.05,;-.78,-3.02,;-4.78,-.71,;-6.19,-1.33,;-7.22,-.19,;-6.45,1.15,;-4.94,.83,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The CD38 enzyme assay was performed as described previously (Becherer, J D, et al. J. Med. Chem. 2015, 58, 7021-7056). Briefly, 200 nL of a dose resp... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z89H88 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data