| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Genome polyprotein |
|---|
| Ligand | BDBM516553 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Measuring Compound Inhibitory Potency |
|---|
| EC50 | 0.010±n/a nM |
|---|
| Citation |  Coburn, CA; Ludmerer, SW; Liu, K; Wu, H; Soil, R; Zhong, B; Zhu, J Inhibitors of hepatitis C virus replication US Patent US11053243 Publication Date 7/6/2021 Coburn, CA; Ludmerer, SW; Liu, K; Wu, H; Soil, R; Zhong, B; Zhu, J Inhibitors of hepatitis C virus replication US Patent US11053243 Publication Date 7/6/2021 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Genome polyprotein |
|---|
| Name: | Genome polyprotein |
|---|
| Synonyms: | HCV Polymerase (S282T) | NS3 serine protease (NS3) | NS3/4A Protein | NS3/4a Protease | POLG_HCVJA |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 327076.78 |
|---|
| Organism: | Hepatitis C Virus (Virus) |
|---|
| Description: | P26662 |
|---|
| Residue: | 3010 |
|---|
| Sequence: | MSTNPKPQRKTKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRG
RRQPIPKARRPEGRTWAQPGYPWPLYGNEGMGWAGWLLSPRGSRPSWGPTDPRRRSRNLG
KVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLA
LLSCLTIPASAYEVRNVSGIYHVTNDCSNSSIVYEAADMIMHTPGCVPCVRESNFSRCWV
ALTPTLAARNSSIPTTTIRRHVDLLVGAAALCSAMYVGDLCGSVFLVSQLFTFSPRRYET
VQDCNCSIYPGHVSGHRMAWDMMMNWSPTTALVVSQLLRIPQAVVDMVAGAHWGVLAGLA
YYSMVGNWAKVLIVMLLFAGVDGHTHVTGGRVASSTQSLVSWLSQGPSQKIQLVNTNGSW
HINRTALNCNDSLQTGFIAALFYAHRFNASGCPERMASCRPIDEFAQGWGPITHDMPESS
DQRPYCWHYAPRPCGIVPASQVCGPVYCFTPSPVVVGTTDRFGAPTYSWGENETDVLLLS
NTRPPQGNWFGCTWMNSTGFTKTCGGPPCNIGGVGNNTLVCPTDCFRKHPEATYTKCGSG
PWLTPRCMVDYPYRLWHYPCTVNFTVFKVRMYVGGVEHRLNAACNWTRGERCDLEDRDRS
ELSPLLLSTTEWQILPCSFTTLPALSTGLIHLHRNIVDVQYLYGIGSAVVSFAIKWEYIL
LLFLLLADARVCACLWMMLLIAQAEATLENLVVLNAASVAGAHGLLSFLVFFCAAWYIKG
RLVPGAAYALYGVWPLLLLLLALPPRAYAMDREMAASCGGAVFVGLVLLTLSPYYKVFLA
RLIWWLQYFITRAEAHLQVWVPPLNVRGGRDAIILLTCAVHPELIFDITKLLLAILGPLM
VLQAGITRVPYFVRAQGLIRACMLVRKVAGGHYVQMAFMKLAALTGTYVYDHLTPLRDWA
HAGLRDLAVAVEPVVFSDMETKLITWGADTAACGDIISGLPVSARRGKEILLGPADSFGE
QGWRLLAPITAYSQQTRGLLGCIITSLTGRDKNQVDGEVQVLSTATQSFLATCVNGVCWT
VYHGAGSKTLAGPKGPITQMYTNVDQDLVGWPAPPGARSMTPCTCGSSDLYLVTRHADVV
PVRRRGDSRGSLLSPRPISYLKGSSGGPLLCPSGHVVGIFRAAVCTRGVAKAVDFIPVES
METTMRSPVFTDNSSPPAVPQTFQVAHLHAPTGSGKSTKVPAAYAAQGYKVLVLNPSVAA
TLGFGAYMSKAHGIEPNIRTGVRTITTGGPITYSTYCKFLADGGCSGGAYDIIICDECHS
TDSTTILGIGTVLDQAETAGARLVVLATATPPGSITVPHPNIEEVALSNTGEIPFYGKAI
PIEAIKGGRHLIFCHSKKKCDELAAKLTGLGLNAVAYYRGLDVSVIPTSGDVVVVATDAL
MTGFTGDFDSVIDCNTCVTQTVDFSLDPTFTIETTTLPQDAVSRAQRRGRTGRGRSGIYR
FVTPGERPSGMFDSSVLCECYDAGCAWYELTPAETSVRLRAYLNTPGLPVCQDHLEFWES
VFTGLTHIDAHFLSQTKQAGDNLPYLVAYQATVCARAQAPPPSWDQMWKCLIRLKPTLHG
PTPLLYRLGAVQNEVTLTHPITKYIMACMSADLEVVTSTWVLVGGVLAALAAYCLTTGSV
VIVGRIILSGRPAVIPDREVLYQEFDEMEECASHLPYIEQGMQLAEQFKQKALGLLQTAT
KQAEAAAPVVESKWRALEVFWAKHMWNFISGIQYLAGLSTLPGNPAIASLMAFTASITSP
LTTQNTLLFNILGGWVAAQLAPPSAASAFVGAGIAGAAVGSIGLGKVLVDILAGYGAGVA
GALVAFKVMSGEMPSTEDLVNLLPAILSPGALVVGVVCAAILRRHVGPGEGAVQWMNRLI
AFASRGNHVSPTHYVPESDAAARVTQILSSLTITQLLKRLHQWINEDCSTPCSGSWLKDV
WDWICTVLSDFKTWLQSKLLPRLPGLPFLSCQRGYKGVWRGDGIMQTTCPCGAQITGHVK
NGSMRIVGPKTCSNTWHGTFPINAYTTGPCTPSPAPNYSRALWRVAAEEYVEVTRVGDFH
YVTGMTTDNVKCPCQVPAPEFFTEVDGVRLHRYAPVCKPLLREEVVFQVGLNQYLVGSQL
PCEPEPDVAVLTSMLTDPSHITAETAKRRLARGSPPSLASSSASQLSAPSLKATCTTHHD
SPDADLIEANLLWRQEMGGNITRVESENKVVILDSFDPIRAVEDEREISVPAEILRKPRK
FPPALPIWARPDYNPPLLESWKDPDYVPPVVHGCPLPSTKAPPIPPPRRKRTVVLTESTV
SSALAELATKTFGSSGSSAVDSGTATGPPDQASDDGDKGSDVESYSSMPPLEGEPGDPDL
SDGSWSTVSGEAGEDVVCCSMSYTWTGALITPCAAEESKLPINPLSNSLLRHHSMVYSTT
SRSASLRQKKVTFDRLQVLDDHYRDVLKEMKAKASTVKARLLSIEEACKLTPPHSAKSKF
GYGAKDVRSLSSRAVNHIRSVWEDLLEDTETPIDTTIMAKNEVFCVQPEKGGRKPARLIV
FPDLGVRVCEKMALYDVVSTLPQAVMGPSYGFQYSPGQRVEFLVNTWKSKKCPMGFSYDT
RCFDSTVTENDIRTEESIYQCCDLAPEARQAIRSLTERLYVGGPLTNSKGQNCGYRRCRA
SGVLTTSCGNTLTCYLKATAACRAAKLQDCTMLVNGDDLVVICESAGTQEDAAALRAFTE
AMTRYSAPPGDPPQPEYDLELITSCSSNVSVAHDASGKRVYYLTRDPTTPLARAAWETVR
HTPVNSWLGNIIMYAPTLWARMILMTHFFSILLAQEQLEKALDCQIYGACYSIEPLDLPQ
IIERLHGLSAFSLHSYSPGEINRVASCLRKLGVPPLRVWRHRARSVRAKLLSQGGRAATC
GKYLFNWAVKTKLKLTPIPAASQLDLSGWFVAGYNGGDIYHSLSRARPRWFMLCLLLLSV
GVGIYLLPNR
|
|
|
|---|
| BDBM516553 |
|---|
| n/a |
|---|
| Name | BDBM516553 |
|---|
| Synonyms: | (2S,2'S)-N,N'-5,6,7,12- tetrahydrobenzo[6,7]cyclohepta[1,2- b]indole-3,9-diylbis{1- [(2R)-2-(dimethylamino)-2- phenylacetyl]pyrrolidine-2- carboxamide} | US11053243, Example 95 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C47H53N7O4 |
|---|
| Mol. Mass. | 779.9682 |
|---|
| SMILES | CN(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)Nc1ccc2-c3[nH]c4ccc(NC(=O)[C@@H]5CCCN5C(=O)[C@H](N(C)C)c5ccccc5)cc4c3CCCc2c1)c1ccccc1 |r| |
|---|
| Structure | 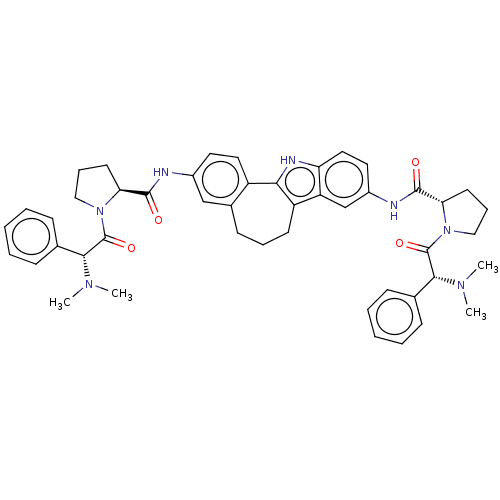
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Coburn, CA; Ludmerer, SW; Liu, K; Wu, H; Soil, R; Zhong, B; Zhu, J Inhibitors of hepatitis C virus replication US Patent US11053243 Publication Date 7/6/2021
Coburn, CA; Ludmerer, SW; Liu, K; Wu, H; Soil, R; Zhong, B; Zhu, J Inhibitors of hepatitis C virus replication US Patent US11053243 Publication Date 7/6/2021