

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50280116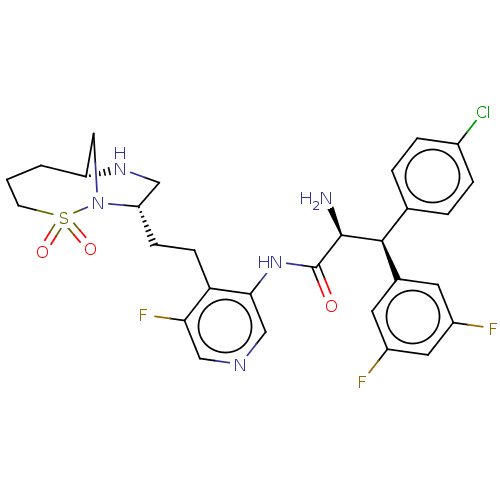 (CHEMBL4177355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190623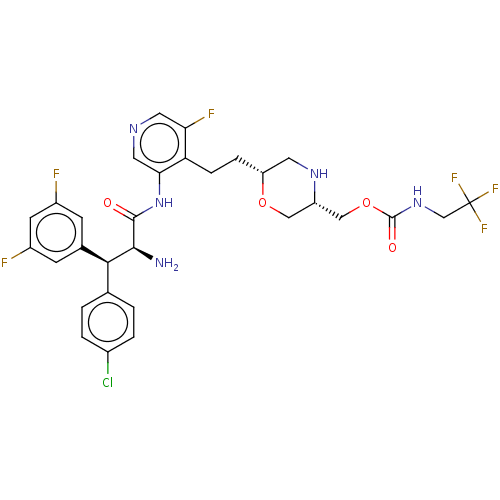 (CHEMBL3828743) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM86731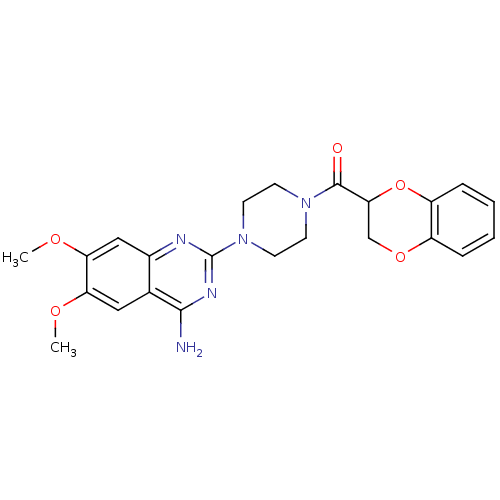 (CAS_74191-85-8 | Doxazosin | UK 33,274) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from rat alpha1A adrenergic receptor after 60 mins by scintillation counting analysis | Eur J Med Chem 143: 1261-1276 (2018) Article DOI: 10.1016/j.ejmech.2017.10.026 BindingDB Entry DOI: 10.7270/Q2D50QG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113626 (CHEMBL3604634) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159537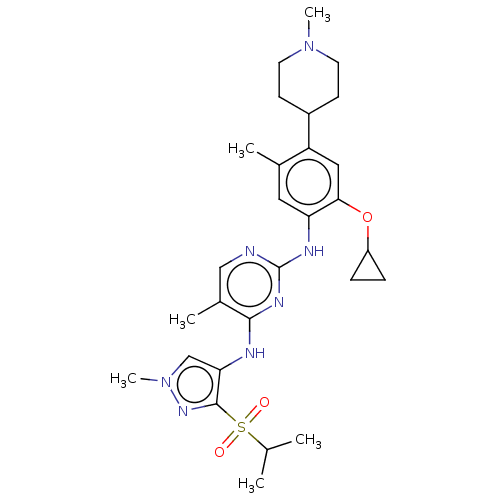 (CHEMBL3785722) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159539 (CHEMBL3787598) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159541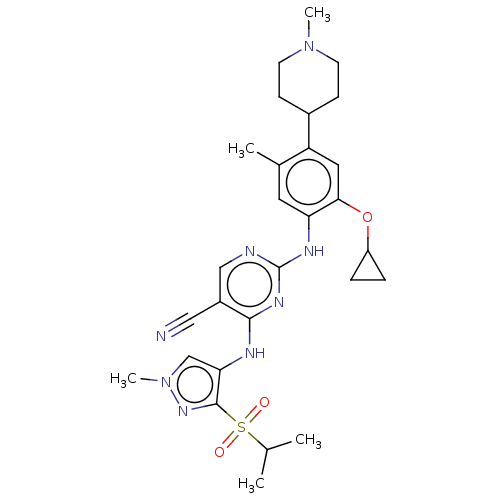 (CHEMBL3785890) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113508 (CHEMBL3604649) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113518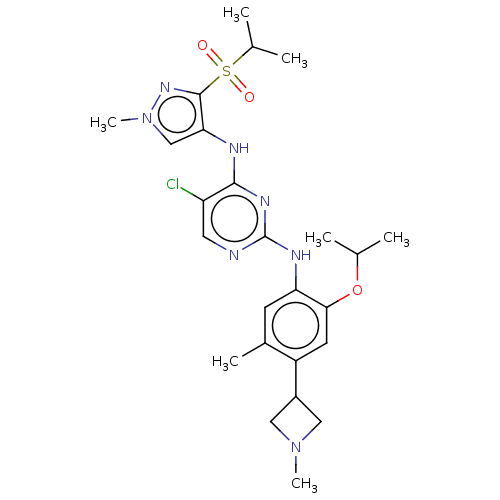 (CHEMBL3604646) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113514 (CHEMBL3604652) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113627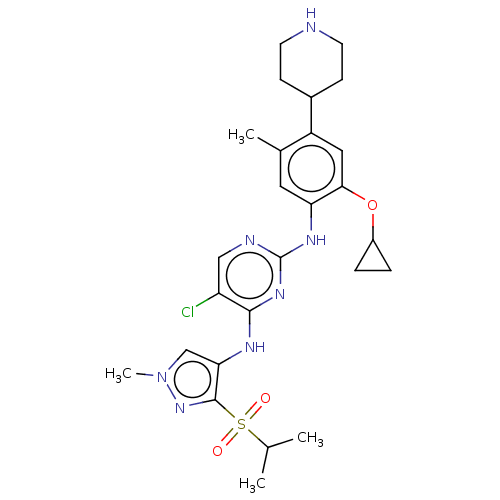 (CHEMBL3604633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113626 (CHEMBL3604634) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50284027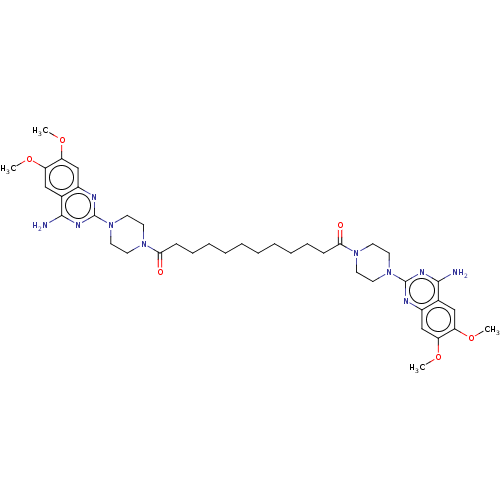 (CHEMBL4170029) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from rat alpha1A adrenergic receptor after 60 mins by scintillation counting analysis | Eur J Med Chem 143: 1261-1276 (2018) Article DOI: 10.1016/j.ejmech.2017.10.026 BindingDB Entry DOI: 10.7270/Q2D50QG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159514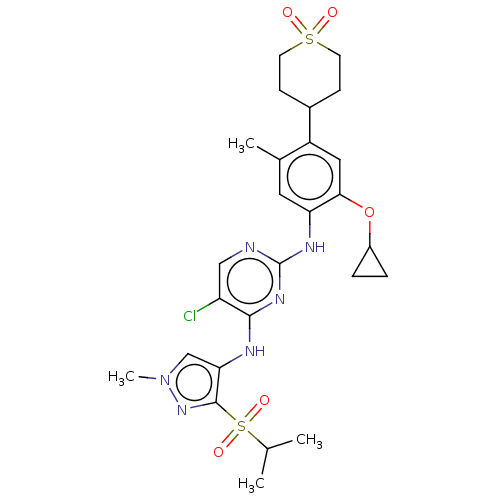 (CHEMBL3785607) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159522 (CHEMBL3785711) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159531 (CHEMBL3786148) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159536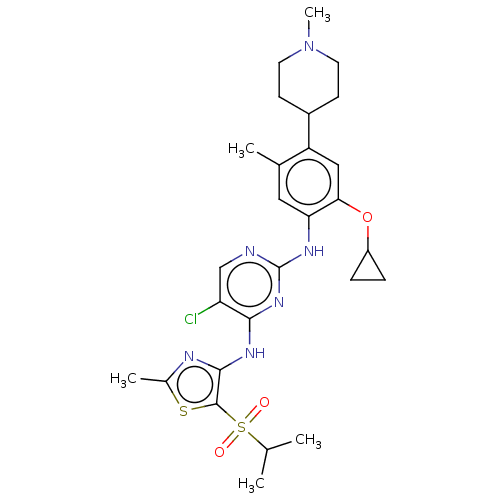 (CHEMBL3786916) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113510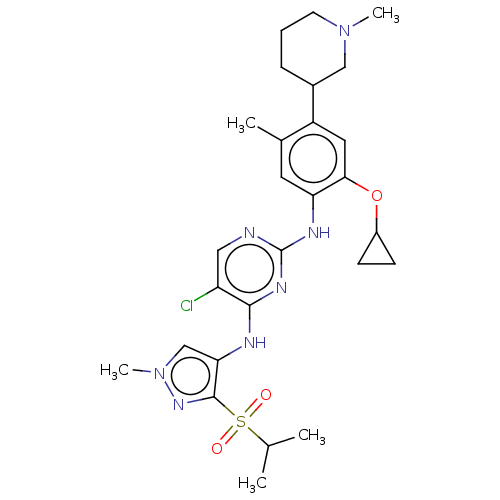 (CHEMBL3604656) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113522 (CHEMBL3604642) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113523 (CHEMBL3604641) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM235541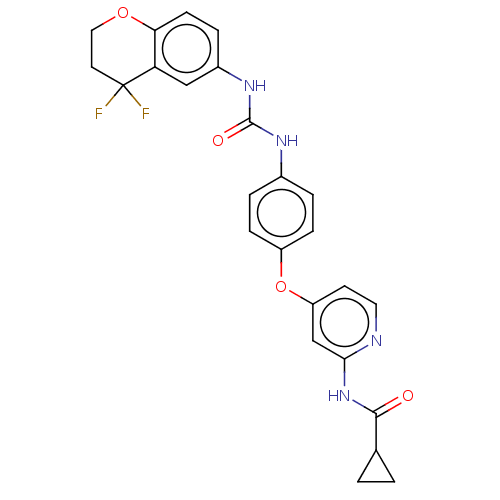 (US9359338, Exe. 151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use ADP-GLO™ assay kit from Promega to evaluate the inhibitory activity of compounds at B-Raf. [Instrument]: PerKinElmer... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM235412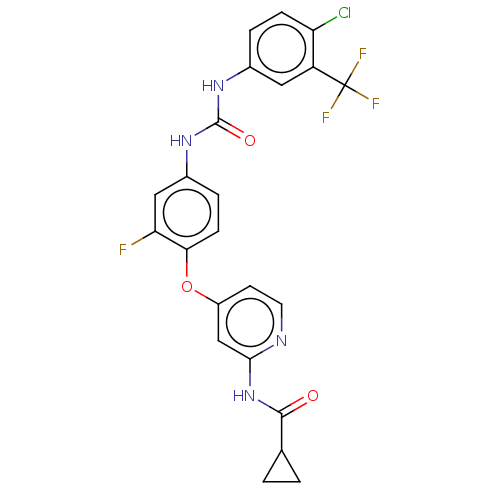 (US9359338, Exe. 022 | US9359338, Exe. 027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use ADP-GLO™ assay kit from Promega to evaluate the inhibitory activity of compounds at B-Raf. [Instrument]: PerKinElmer... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM235627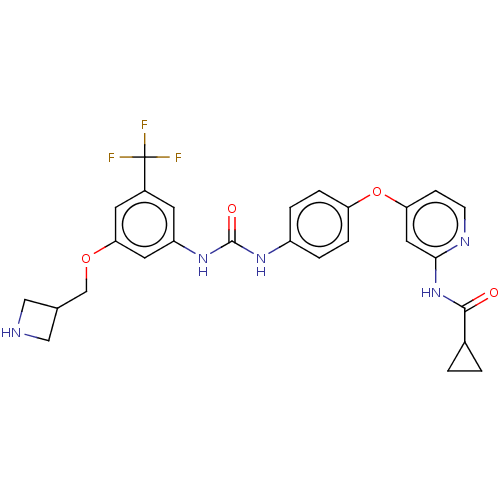 (US9359338, Exe. 237) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use ADP-GLO™ assay kit from Promega to evaluate the inhibitory activity of compounds at B-Raf. [Instrument]: PerKinElmer... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235451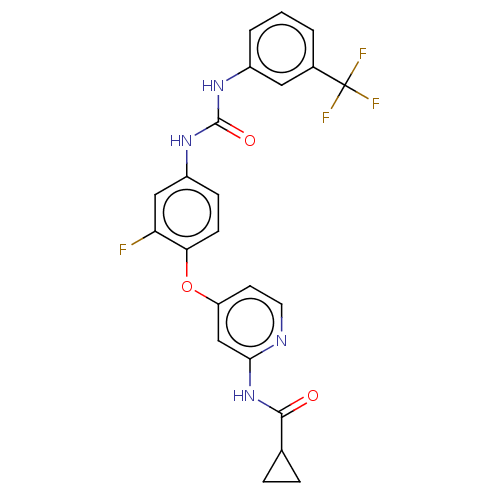 (US9359338, Exe. 061) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.73 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235419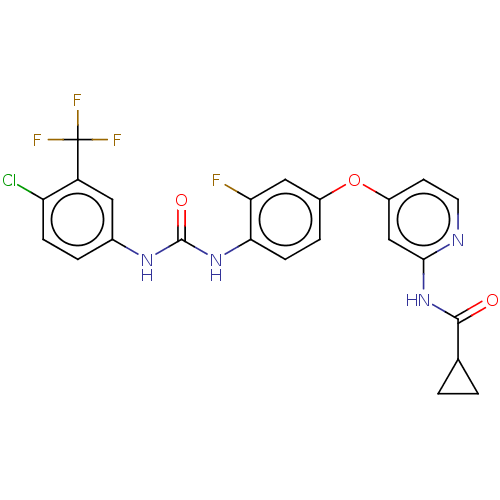 (US9359338, Exe. 029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113626 (CHEMBL3604634) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK L1196M mutant using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159541 (CHEMBL3785890) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK L1196M mutant using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113517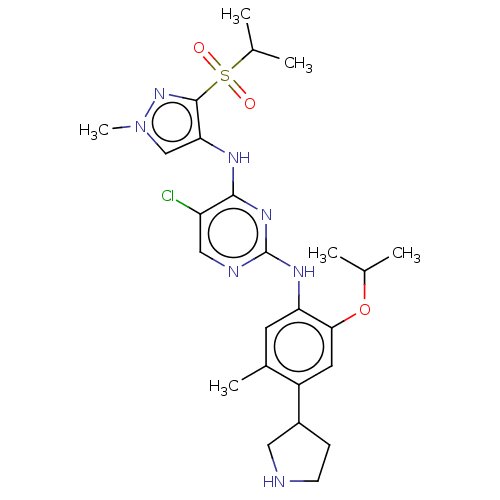 (CHEMBL3604647) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113519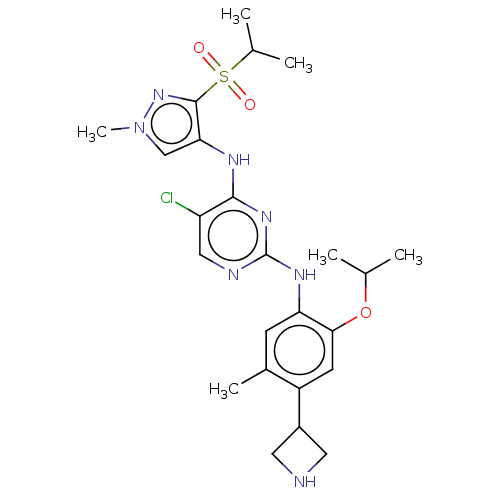 (CHEMBL3604645) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113521 (CHEMBL3604643) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113629 (CHEMBL3604632) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113516 (CHEMBL3604650) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113511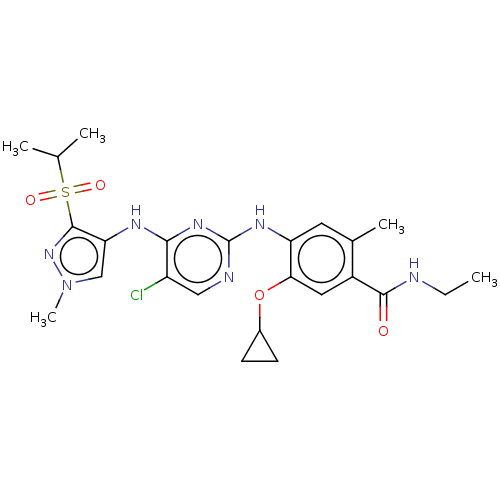 (CHEMBL3604655) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113627 (CHEMBL3604633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113626 (CHEMBL3604634) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113507 (CHEMBL3604648) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50113522 (CHEMBL3604642) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay | Bioorg Med Chem Lett 25: 3738-43 (2015) Article DOI: 10.1016/j.bmcl.2015.06.021 BindingDB Entry DOI: 10.7270/Q208674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235405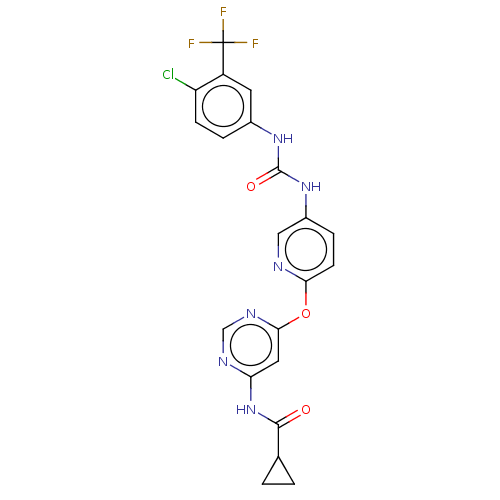 (US9359338, Exe. 015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235431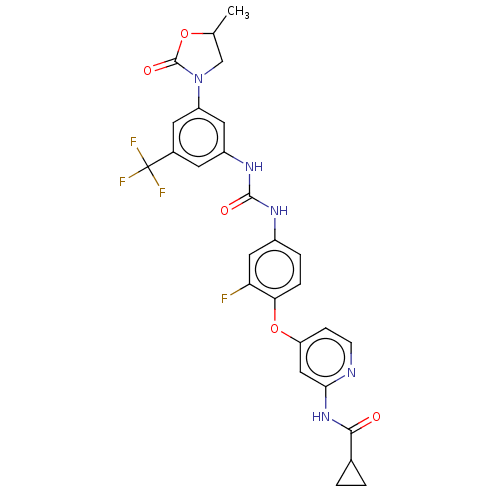 (US9359338, Exe. 041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235412 (US9359338, Exe. 022 | US9359338, Exe. 027) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50284026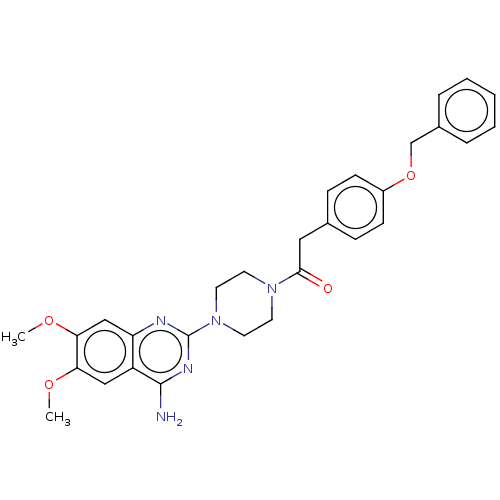 (CHEMBL4166644) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from rat alpha1A adrenergic receptor after 60 mins by scintillation counting analysis | Eur J Med Chem 143: 1261-1276 (2018) Article DOI: 10.1016/j.ejmech.2017.10.026 BindingDB Entry DOI: 10.7270/Q2D50QG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235541 (US9359338, Exe. 151) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235520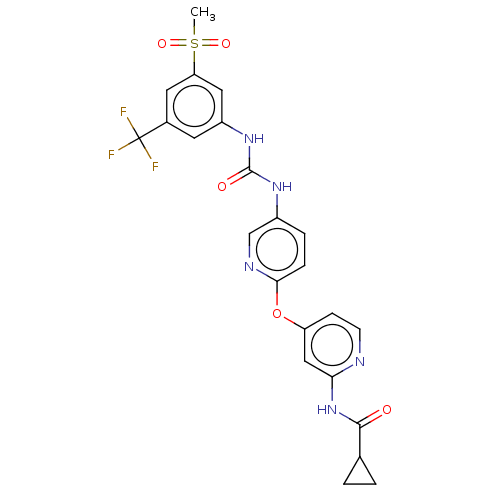 (US9359338, Exe. 130) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM235454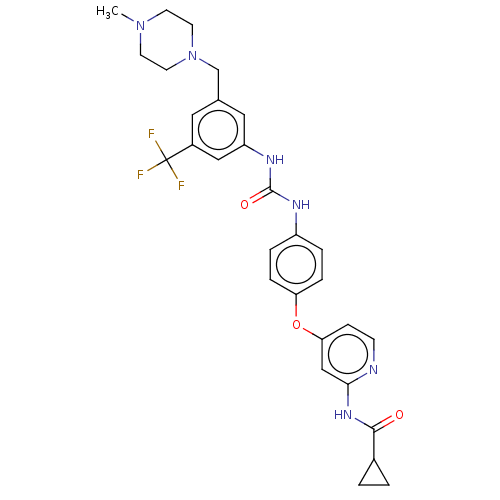 (US9359338, Exe. 064) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use ADP-GLO™ assay kit from Promega to evaluate the inhibitory activity of compounds at B-Raf. [Instrument]: PerKinElmer... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM235571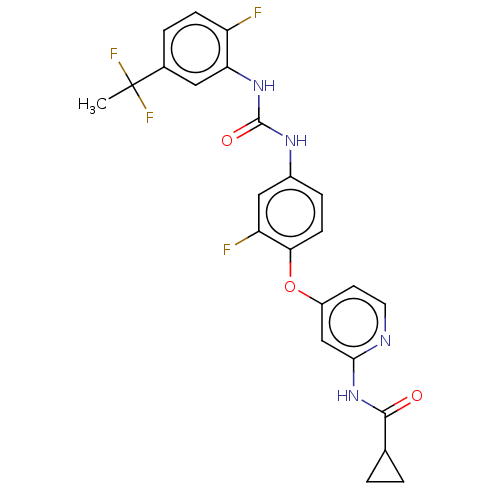 (US9359338, Exe. 181) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use ADP-GLO™ assay kit from Promega to evaluate the inhibitory activity of compounds at B-Raf. [Instrument]: PerKinElmer... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 [790-1356] (Homo sapiens (Human)) | BDBM235565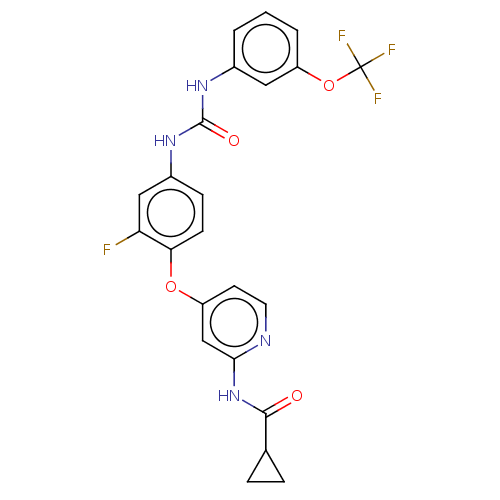 (US9359338, Exe. 175) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | 30 |
CROWN BIOSCIENCE INC. (Taiwan) US Patent | Assay Description [Experimental method]: Use Lance@Ultra Ulight™-TK assay kit from PerKinElmer to evaluate the inhibitory activity of compounds at KDR kinase. [I... | US Patent US9359338 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159513 (CHEMBL3785825) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50159517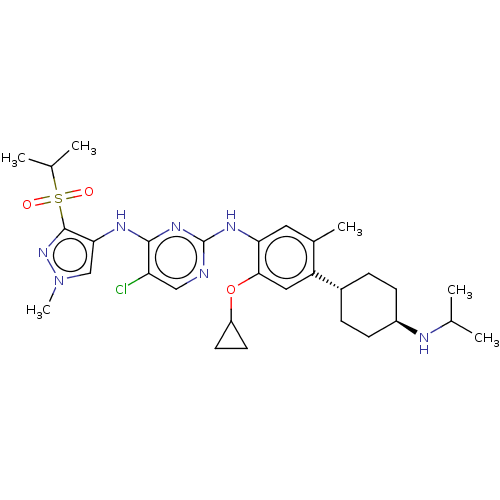 (CHEMBL3785928) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1910-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.017 BindingDB Entry DOI: 10.7270/Q26M38QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 773 total ) | Next | Last >> |