

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Hepatocyte growth factor receptor [D1228H] | ||
| Ligand | BDBM516967 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Enzyme Assay | ||
| IC50 | 6.10±n/a nM | ||
| Citation |  Hinklin, RJ; Allen, S; Barbour, P; Cook, A; Dahlke, J; Gaudino, J; Laird, E; McNulty, OT; Zhao, Q Pyrazolo[3,4-b]pyridine compounds as inhibitors of TAM and MET kinases US Patent US11104676 Publication Date 8/31/2021 Hinklin, RJ; Allen, S; Barbour, P; Cook, A; Dahlke, J; Gaudino, J; Laird, E; McNulty, OT; Zhao, Q Pyrazolo[3,4-b]pyridine compounds as inhibitors of TAM and MET kinases US Patent US11104676 Publication Date 8/31/2021 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Hepatocyte growth factor receptor [D1228H] | |||
| Name: | Hepatocyte growth factor receptor [D1228H] | ||
| Synonyms: | Hepatocyte growth factor receptor (MET)(D1228H) | MET | MET_HUMAN | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 155583.30 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P08581[D1228H] | ||
| Residue: | 1390 | ||
| Sequence: |
| ||
| BDBM516967 | |||
| n/a | |||
| Name | BDBM516967 | ||
| Synonyms: | N-(3-fluoro-4-((3- (((1s,3s)-3- (hydroxymethyl)cyclobutyl) amino)-1H- pyrazolo[3,4-b]pyridin-4- yl)oxy)phenyl)-2-(4- fluorophenyl)-3-oxo-2,3- dihydropyridazine-4- carboxamide | US11104676, Example 37 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C28H23F2N7O4 | ||
| Mol. Mass. | 559.5235 | ||
| SMILES | OC[C@H]1CC(C1)Nc1n[nH]c2nccc(Oc3ccc(NC(=O)c4ccnn(-c5ccc(F)cc5)c4=O)cc3F)c12 |r,wU:2.1,(-12.34,2.12,;-11.94,.63,;-10.45,.23,;-9.12,1,;-8.35,-.33,;-9.68,-1.1,;-6.86,-.73,;-6.46,-2.22,;-7.37,-3.47,;-6.46,-4.71,;-5,-4.23,;-3.67,-5,;-2.33,-4.23,;-2.33,-2.69,;-3.67,-1.93,;-3.67,-.38,;-2.33,.38,;-1,-.38,;.34,.38,;.34,1.93,;1.67,2.69,;3,1.93,;3,.38,;4.34,2.69,;4.34,4.23,;5.67,5,;7,4.23,;7,2.69,;8.34,1.93,;8.34,.38,;9.67,-.38,;11.01,.38,;12.34,-.38,;11.01,1.93,;9.67,2.69,;5.67,1.93,;5.67,.38,;-1,2.69,;-2.33,1.93,;-3.67,2.69,;-5,-2.69,)| | ||
| Structure | 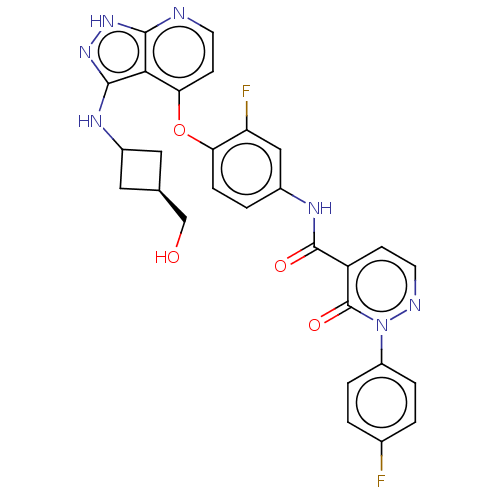
| ||