| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Metallo-beta-lactamase type 2 |
|---|
| Ligand | BDBM531027 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Enzyme Activity: Determination of IC50 |
|---|
| IC50 | 0.610±n/a nM |
|---|
| Citation |  Pasternak, A; Dong, S; Scott, JD; Tang, H; Zhao, Z; Yang, D; Gu, X; Jiang, J; Xiao, L Metallo-beta-lactamase inhibitors and methods of use thereof US Patent US11207312 Publication Date 12/28/2021 Pasternak, A; Dong, S; Scott, JD; Tang, H; Zhao, Z; Yang, D; Gu, X; Jiang, J; Xiao, L Metallo-beta-lactamase inhibitors and methods of use thereof US Patent US11207312 Publication Date 12/28/2021 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Metallo-beta-lactamase type 2 |
|---|
| Name: | Metallo-beta-lactamase type 2 |
|---|
| Synonyms: | B2 metallo-beta-lactamase | BLAN1_KLEPN | Beta-lactamase NDM-1 | Beta-lactamase type II | Metallo-beta-lactamase NDM-1 | Metallo-beta-lactamase type 2 | Metallo-beta-lactamase type II | NDM-1 | New Delhi metallo-beta-lactamase-1 | blaNDM-1 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 28498.15 |
|---|
| Organism: | Klebsiella pneumoniae |
|---|
| Description: | ChEMBL_103887 |
|---|
| Residue: | 270 |
|---|
| Sequence: | MELPNIMHPVAKLSTALAAALMLSGCMPGEIRPTIGQQMETGDQRFGDLVFRQLAPNVWQ
HTSYLDMPGFGAVASNGLIVRDGGRVLVVDTAWTDDQTAQILNWIKQEINLPVALAVVTH
AHQDKMGGMDALHAAGIATYANALSNQLAPQEGMVAAQHSLTFAANGWVEPATAPNFGPL
KVFYPGPGHTSDNITVGIDGTDIAFGGCLIKDSKAKSLGNLGDADTEHYAASARAFGAAF
PKASMIVMSHSAPDSRAAITHTARMADKLR
|
|
|
|---|
| BDBM531027 |
|---|
| n/a |
|---|
| Name | BDBM531027 |
|---|
| Synonyms: | 4-(4-hydroxy-2-oxopiperidin-1- yl)-N1-((R)-pyrrolidin-3-yl)-3- (2H-tetrazol-5-yl)benzene-1,2- disulfonamide | US11207312, Example 159 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H22N8O6S2 |
|---|
| Mol. Mass. | 486.526 |
|---|
| SMILES | NS(=O)(=O)c1c(ccc(N2CCC(O)CC2=O)c1-c1nn[nH]n1)S(=O)(=O)N[C@@H]1CCNC1 |r| |
|---|
| Structure | 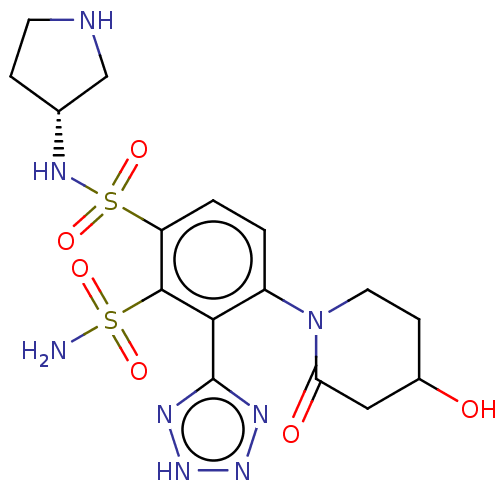
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pasternak, A; Dong, S; Scott, JD; Tang, H; Zhao, Z; Yang, D; Gu, X; Jiang, J; Xiao, L Metallo-beta-lactamase inhibitors and methods of use thereof US Patent US11207312 Publication Date 12/28/2021
Pasternak, A; Dong, S; Scott, JD; Tang, H; Zhao, Z; Yang, D; Gu, X; Jiang, J; Xiao, L Metallo-beta-lactamase inhibitors and methods of use thereof US Patent US11207312 Publication Date 12/28/2021