 Found 8858 hits with Last Name = 'xiao' and Initial = 'l'
Found 8858 hits with Last Name = 'xiao' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463294

(CHEMBL4249256)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@H](C(=O)c4ccccc4)[C@]2(OC)C=C1)ccc3OC |r,wU:16.16,1.0,wD:17.38,28.36,7.7,19.23,c:37,THB:10:9:17:5.6.4,(9.78,-11.07,;9.03,-9.74,;7.65,-10.81,;5.94,-9.74,;6.72,-8.4,;5.95,-7.07,;6.71,-5.74,;8.25,-5.74,;9.79,-5.74,;9.03,-4.41,;9.78,-3.07,;11.32,-3.05,;12.66,-3.81,;12.64,-2.27,;7.47,-5.14,;7.41,-7.18,;8.25,-8.41,;9.02,-7.07,;10.56,-7.06,;11.34,-8.4,;12.88,-8.4,;13.65,-7.07,;13.65,-9.73,;12.87,-11.06,;13.64,-12.4,;15.18,-12.4,;15.95,-11.05,;15.18,-9.72,;10.59,-9.7,;11.34,-11.07,;10.57,-12.41,;9.25,-8.93,;10.35,-7.84,;4.41,-7.06,;3.64,-8.38,;4.4,-9.72,;3.62,-11.05,;2.08,-11.04,)| Show InChI InChI=1S/C31H33NO4/c1-34-23-11-10-21-16-24-29-12-13-31(35-2,22(17-29)26(33)20-6-4-3-5-7-20)28-30(29,25(21)27(23)36-28)14-15-32(24)18-19-8-9-19/h3-7,10-13,19,22,24,28H,8-9,14-18H2,1-2H3/t22-,24-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506108

(CHEMBL4449252)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(=O)c2ccccc2)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C37H40N2O4/c1-41-29-15-12-26-20-30-35-16-17-37(42-2,34-36(35,31(26)32(29)43-34)18-19-39(30)22-23-8-9-23)28(21-35)24-10-13-27(14-11-24)38-33(40)25-6-4-3-5-7-25/h3-7,10-15,23,28,30,34H,8-9,16-22H2,1-2H3,(H,38,40)/t28-,30-,34-,35-,36+,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362726
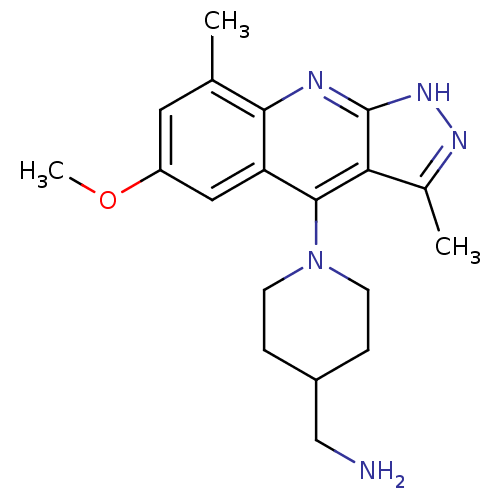
(CHEMBL1939796)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(CN)CC3)c2c1 Show InChI InChI=1S/C19H25N5O/c1-11-8-14(25-3)9-15-17(11)21-19-16(12(2)22-23-19)18(15)24-6-4-13(10-20)5-7-24/h8-9,13H,4-7,10,20H2,1-3H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362722
(CHEMBL1939800)Show InChI InChI=1S/C19H19N5O/c1-11-8-14(25-3)9-15-17(11)22-19-16(12(2)23-24-19)18(15)21-10-13-4-6-20-7-5-13/h4-9H,10H2,1-3H3,(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362037

(CHEMBL1939916)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(CN3CCOC(CO)C3)c2c1 Show InChI InChI=1S/C19H24N4O3/c1-11-6-13(25-3)7-15-16(9-23-4-5-26-14(8-23)10-24)17-12(2)21-22-19(17)20-18(11)15/h6-7,14,24H,4-5,8-10H2,1-3H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50520722
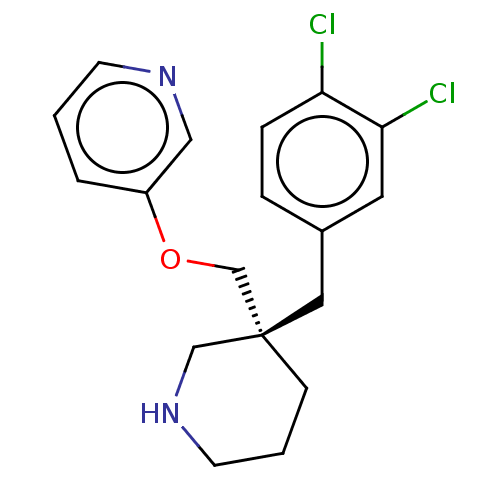
(CHEMBL4554047)Show SMILES Clc1ccc(C[C@@]2(COc3cccnc3)CCCNC2)cc1Cl |r| Show InChI InChI=1S/C18H20Cl2N2O/c19-16-5-4-14(9-17(16)20)10-18(6-2-8-22-12-18)13-23-15-3-1-7-21-11-15/h1,3-5,7,9,11,22H,2,6,8,10,12-13H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis acetylcholine-binding protein incubated for 60 mins followed by 3 hrs incubation in dark condi... |
Eur J Med Chem 160: 37-48 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.073
BindingDB Entry DOI: 10.7270/Q2QV3QXP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393757
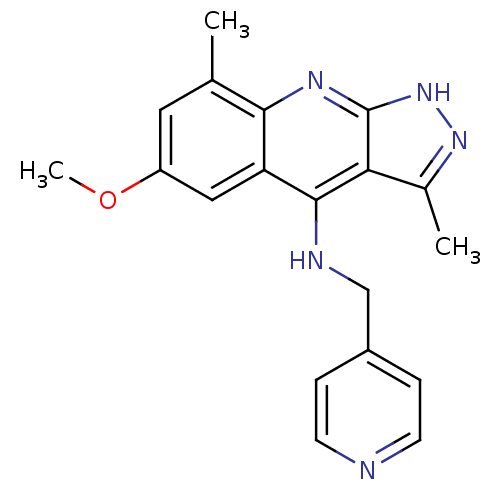
(CHEMBL2159143)Show InChI InChI=1S/C17H20N6/c1-12-21-15-11-20-17-14(3-7-19-17)16(15)23(12)13-4-9-22(10-5-13)8-2-6-18/h3,7,11,13H,2,4-5,8-10H2,1H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362728
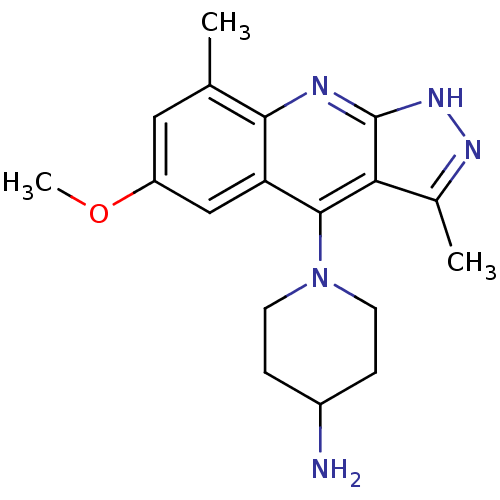
(CHEMBL1939794)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(N)CC3)c2c1 Show InChI InChI=1S/C18H23N5O/c1-10-8-13(24-3)9-14-16(10)20-18-15(11(2)21-22-18)17(14)23-6-4-12(19)5-7-23/h8-9,12H,4-7,19H2,1-3H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362035
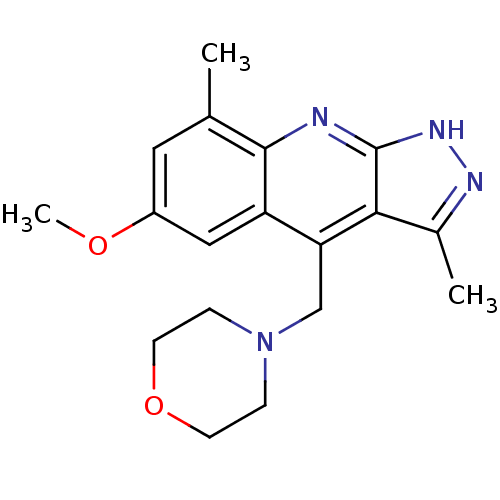
(CHEMBL1939914)Show InChI InChI=1S/C18H22N4O2/c1-11-8-13(23-3)9-14-15(10-22-4-6-24-7-5-22)16-12(2)20-21-18(16)19-17(11)14/h8-9H,4-7,10H2,1-3H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362047
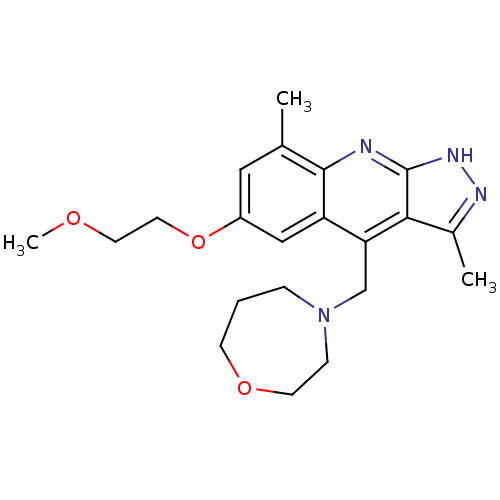
(CHEMBL1940057)Show SMILES COCCOc1cc(C)c2nc3[nH]nc(C)c3c(CN3CCCOCC3)c2c1 Show InChI InChI=1S/C21H28N4O3/c1-14-11-16(28-10-9-26-3)12-17-18(13-25-5-4-7-27-8-6-25)19-15(2)23-24-21(19)22-20(14)17/h11-12H,4-10,13H2,1-3H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393753
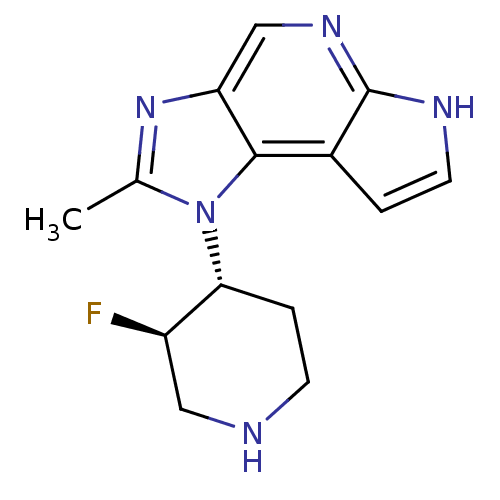
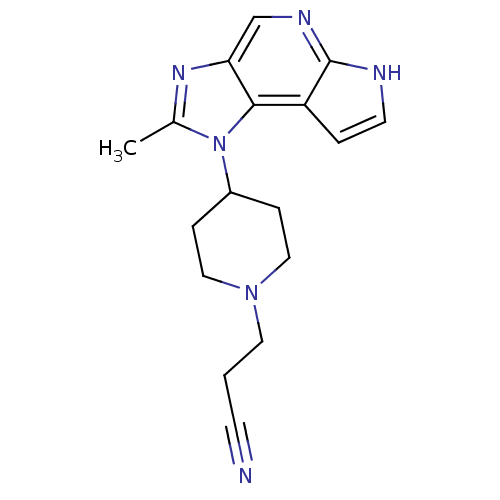
(CHEMBL2159202)Show SMILES Cc1nc2cnc3[nH]ccc3c2n1[C@@H]1CCNC[C@H]1F |r| Show InChI InChI=1S/C14H16FN5/c1-8-19-11-7-18-14-9(2-5-17-14)13(11)20(8)12-3-4-16-6-10(12)15/h2,5,7,10,12,16H,3-4,6H2,1H3,(H,17,18)/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393739
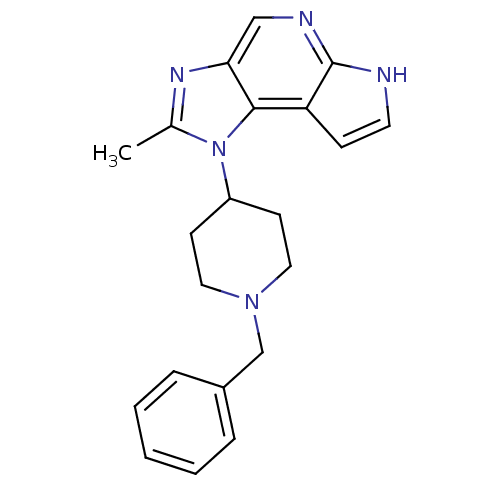
(CHEMBL2159142)Show InChI InChI=1S/C21H23N5/c1-15-24-19-13-23-21-18(7-10-22-21)20(19)26(15)17-8-11-25(12-9-17)14-16-5-3-2-4-6-16/h2-7,10,13,17H,8-9,11-12,14H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393756
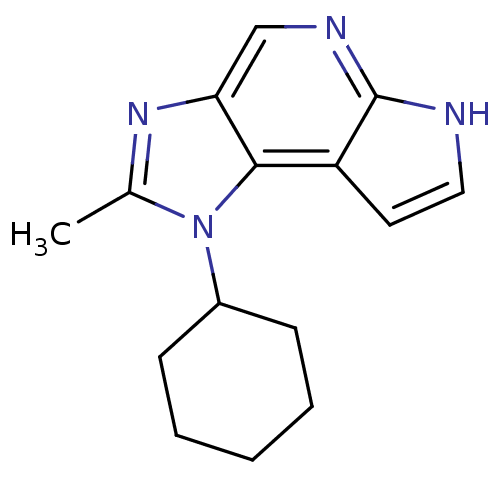
(CHEMBL2159198)Show InChI InChI=1S/C15H18N4/c1-10-18-13-9-17-15-12(7-8-16-15)14(13)19(10)11-5-3-2-4-6-11/h7-9,11H,2-6H2,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50380636

(CHEMBL2017060)Show SMILES COc1cc2CCn3cnc(-c4cnc(s4)C(=O)N4CCCOCC4)c3-c2cc1OC Show InChI InChI=1S/C22H24N4O4S/c1-28-16-10-14-4-6-26-13-24-19(20(26)15(14)11-17(16)29-2)18-12-23-21(31-18)22(27)25-5-3-8-30-9-7-25/h10-13H,3-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as inhibition of [3H]cAMP hydrolysis by scintillation proximity assay |
Bioorg Med Chem Lett 22: 2585-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.113
BindingDB Entry DOI: 10.7270/Q2319WX5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393750
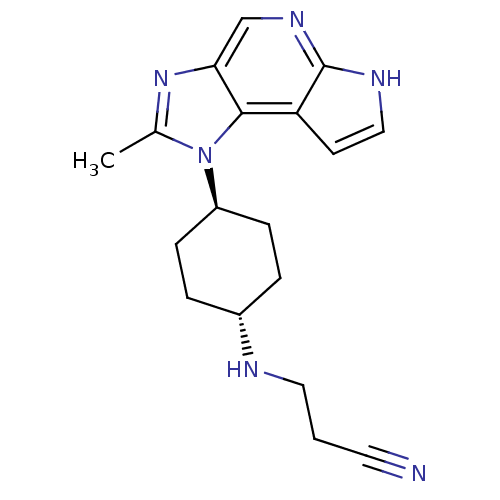
(CHEMBL2159205)Show SMILES Cc1nc2cnc3[nH]ccc3c2n1[C@H]1CC[C@@H](CC1)NCCC#N |r,wU:16.22,wD:13.15,(11.06,-.77,;11.78,-2.13,;11.1,-3.52,;12.21,-4.59,;12.16,-6.13,;13.46,-6.94,;14.82,-6.22,;16.27,-6.76,;17.22,-5.54,;16.36,-4.26,;14.88,-4.68,;13.57,-3.87,;13.31,-2.35,;14.65,-1.58,;15.98,-2.35,;17.31,-1.58,;17.31,-.04,;15.98,.73,;14.64,-.04,;18.65,.73,;18.64,2.27,;17.31,3.04,;17.31,4.58,;17.31,6.12,)| Show InChI InChI=1S/C18H22N6/c1-12-23-16-11-22-18-15(7-10-21-18)17(16)24(12)14-5-3-13(4-6-14)20-9-2-8-19/h7,10-11,13-14,20H,2-6,9H2,1H3,(H,21,22)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393744
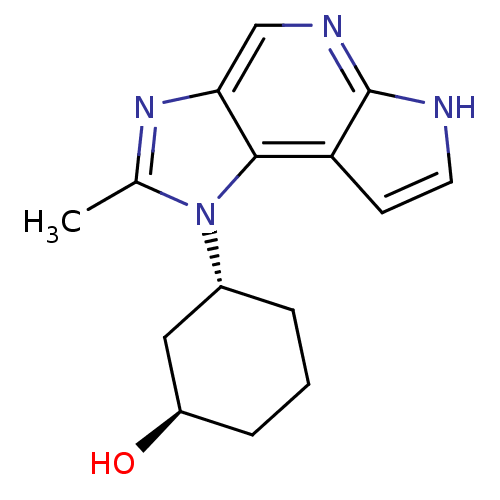
(CHEMBL2159210)Show SMILES Cc1nc2cnc3[nH]ccc3c2n1[C@@H]1CCC[C@@H](O)C1 |r| Show InChI InChI=1S/C15H18N4O/c1-9-18-13-8-17-15-12(5-6-16-15)14(13)19(9)10-3-2-4-11(20)7-10/h5-6,8,10-11,20H,2-4,7H2,1H3,(H,16,17)/t10-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362046

(CHEMBL1940056)Show InChI InChI=1S/C18H22N4O2/c1-11-8-13(23)9-14-15(10-22-4-3-6-24-7-5-22)16-12(2)20-21-18(16)19-17(11)14/h8-9,23H,3-7,10H2,1-2H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393751

(CHEMBL2159204)Show InChI InChI=1S/C14H15F2N5/c1-8-20-10-6-19-13-9(2-5-18-13)12(10)21(8)11-3-4-17-7-14(11,15)16/h2,5-6,11,17H,3-4,7H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463293
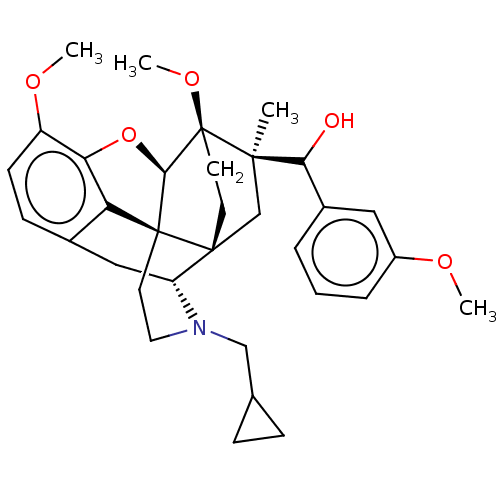
(CHEMBL4242847)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1cccc(OC)c1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)22-6-5-7-23(16-22)36-2)19-31-12-13-33(30,38-4)29-32(31)14-15-34(18-20-8-9-20)25(31)17-21-10-11-24(37-3)27(39-29)26(21)32/h5-7,10-11,16,20,25,28-29,35H,8-9,12-15,17-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50393757

(CHEMBL2159143)Show InChI InChI=1S/C17H20N6/c1-12-21-15-11-20-17-14(3-7-19-17)16(15)23(12)13-4-9-22(10-5-13)8-2-6-18/h3,7,11,13H,2,4-5,8-10H2,1H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50520723
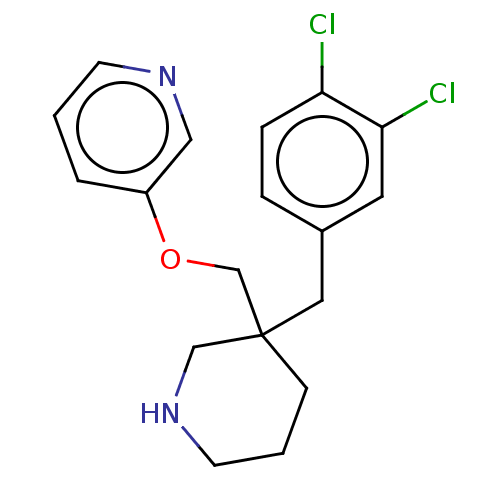
(CHEMBL4440256)Show InChI InChI=1S/C18H20Cl2N2O/c19-16-5-4-14(9-17(16)20)10-18(6-2-8-22-12-18)13-23-15-3-1-7-21-11-15/h1,3-5,7,9,11,22H,2,6,8,10,12-13H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis acetylcholine-binding protein incubated for 60 mins followed by 3 hrs incubation in dark condi... |
Eur J Med Chem 160: 37-48 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.073
BindingDB Entry DOI: 10.7270/Q2QV3QXP |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458670

(CHEMBL4212838)Show SMILES [H][C@@]12CCC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1cc(Cl)ccc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C27H26ClF3N2O5/c28-16-6-11-20-22(14-16)33(26(37)15-4-9-18(10-5-15)38-27(29,30)31)21-3-1-2-19(21)25(20)32(17-7-8-17)23(34)12-13-24(35)36/h4-6,9-11,14,17,19,21,25H,1-3,7-8,12-13H2,(H,35,36)/t19-,21+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458673

(CHEMBL4207731)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1ccccc1[C@@H]2N(C1CC1)C(=O)OCC(O)=O |r| Show InChI InChI=1S/C25H23F3N2O6/c26-25(27,28)36-16-9-5-14(6-10-16)23(33)30-19-4-2-1-3-17(19)22(18-11-12-20(18)30)29(15-7-8-15)24(34)35-13-21(31)32/h1-6,9-10,15,18,20,22H,7-8,11-13H2,(H,31,32)/t18-,20+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393747
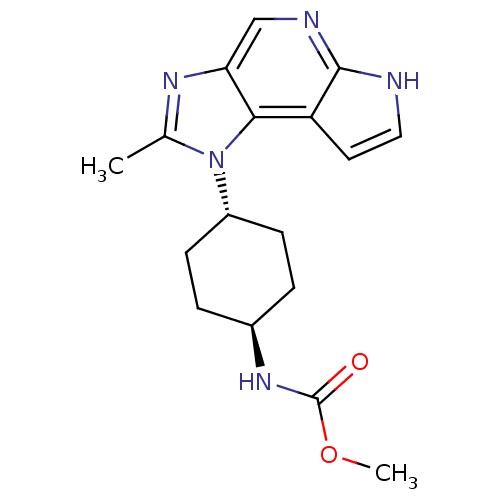
(CHEMBL2159208)Show SMILES COC(=O)N[C@H]1CC[C@@H](CC1)n1c(C)nc2cnc3[nH]ccc3c12 |r,wU:5.4,wD:8.11,(17.33,-10.61,;15.8,-10.54,;14.97,-11.83,;13.43,-11.76,;15.74,-13.16,;14.98,-14.5,;15.73,-15.84,;14.96,-17.17,;13.42,-17.16,;12.65,-15.81,;13.44,-14.49,;12.63,-18.48,;11.09,-18.35,;10.3,-17.03,;10.49,-19.77,;11.66,-20.78,;11.69,-22.31,;13.03,-23.07,;14.35,-22.27,;15.82,-22.72,;16.71,-21.46,;15.78,-20.23,;14.32,-20.73,;12.97,-19.98,)| Show InChI InChI=1S/C17H21N5O2/c1-10-20-14-9-19-16-13(7-8-18-16)15(14)22(10)12-5-3-11(4-6-12)21-17(23)24-2/h7-9,11-12H,3-6H2,1-2H3,(H,18,19)(H,21,23)/t11-,12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463284
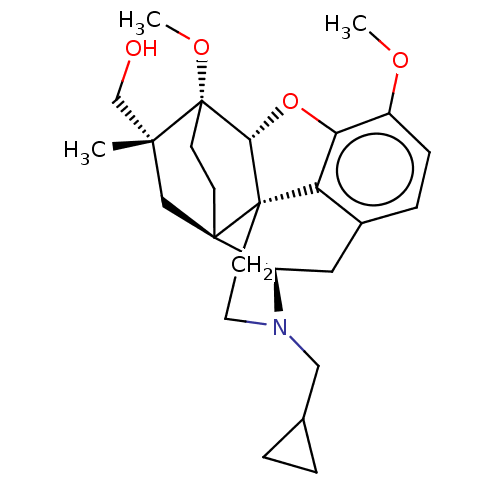
(CHEMBL4245818)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(CO)C1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C26H35NO4/c1-23(15-28)14-24-8-9-26(23,30-3)22-25(24)10-11-27(13-16-4-5-16)19(24)12-17-6-7-18(29-2)21(31-22)20(17)25/h6-7,16,19,22,28H,4-5,8-15H2,1-3H3/t19-,22-,23-,24-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50393754

(CHEMBL2159200)Show InChI InChI=1S/C14H16N4O/c1-9-17-12-7-16-14-11(4-5-15-14)13(12)18(9)10-3-2-6-19-8-10/h4-5,7,10H,2-3,6,8H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50393739

(CHEMBL2159142)Show InChI InChI=1S/C21H23N5/c1-15-24-19-13-23-21-18(7-10-22-21)20(19)26(15)17-8-11-25(12-9-17)14-16-5-3-2-4-6-16/h2-7,10,13,17H,8-9,11-12,14H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458666

(CHEMBL4204359)Show SMILES OC(=O)CCC(=O)N(C1CC1)C1C2CCCC2N(C(=O)OCc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H30N2O5/c30-24(15-16-25(31)32)28(19-13-14-19)26-20-9-4-5-11-22(20)29(23-12-6-10-21(23)26)27(33)34-17-18-7-2-1-3-8-18/h1-5,7-9,11,19,21,23,26H,6,10,12-17H2,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458667

(CHEMBL4207935)Show SMILES [H][C@@]12CCC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1cc(F)ccc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C27H26F4N2O5/c28-16-6-11-20-22(14-16)33(26(37)15-4-9-18(10-5-15)38-27(29,30)31)21-3-1-2-19(21)25(20)32(17-7-8-17)23(34)12-13-24(35)36/h4-6,9-11,14,17,19,21,25H,1-3,7-8,12-13H2,(H,35,36)/t19-,21+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362036

(CHEMBL1939915)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(CN3CCOC(C)C3)c2c1 Show InChI InChI=1S/C19H24N4O2/c1-11-7-14(24-4)8-15-16(10-23-5-6-25-12(2)9-23)17-13(3)21-22-19(17)20-18(11)15/h7-8,12H,5-6,9-10H2,1-4H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362033

(CHEMBL1939912)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(CN3CC[C@@H](O)C3)c2c1 |r| Show InChI InChI=1S/C18H22N4O2/c1-10-6-13(24-3)7-14-15(9-22-5-4-12(23)8-22)16-11(2)20-21-18(16)19-17(10)14/h6-7,12,23H,4-5,8-9H2,1-3H3,(H,19,20,21)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362725
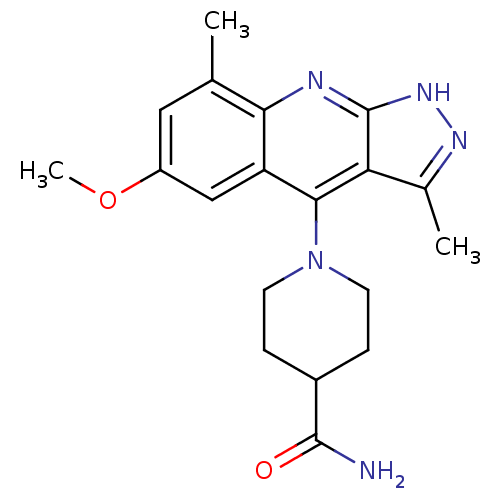
(CHEMBL1939797)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(CC3)C(N)=O)c2c1 Show InChI InChI=1S/C19H23N5O2/c1-10-8-13(26-3)9-14-16(10)21-19-15(11(2)22-23-19)17(14)24-6-4-12(5-7-24)18(20)25/h8-9,12H,4-7H2,1-3H3,(H2,20,25)(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362711

(CHEMBL1939812)Show InChI InChI=1S/C18H21N3O3/c1-10-8-13(22-3)9-14-16(10)19-18-15(11(2)20-21-18)17(14)24-12-4-6-23-7-5-12/h8-9,12H,4-7H2,1-3H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 kinase domain using N-terminal 5-carboxyfluorescein-tagged Leu-Pro-Leu-Asp-Lys-Asp-Tyr-Tyr-Val-Val-Arg as substrate after 30 mins |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362730
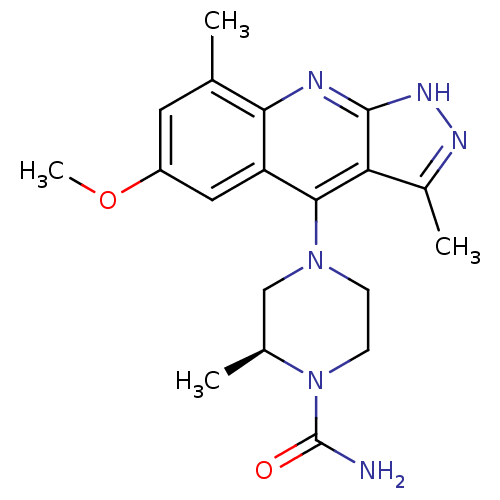
(CHEMBL1939792)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCN([C@@H](C)C3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C19H24N6O2/c1-10-7-13(27-4)8-14-16(10)21-18-15(12(3)22-23-18)17(14)24-5-6-25(19(20)26)11(2)9-24/h7-8,11H,5-6,9H2,1-4H3,(H2,20,26)(H,21,22,23)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50520715
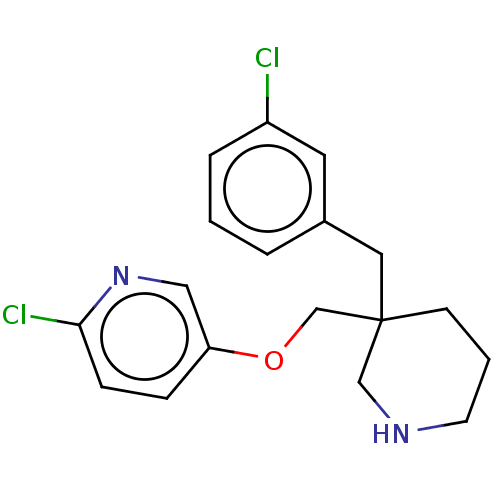
(CHEMBL4437999)Show InChI InChI=1S/C18H20Cl2N2O/c19-15-4-1-3-14(9-15)10-18(7-2-8-21-12-18)13-23-16-5-6-17(20)22-11-16/h1,3-6,9,11,21H,2,7-8,10,12-13H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis acetylcholine-binding protein incubated for 60 mins followed by 3 hrs incubation in dark condi... |
Eur J Med Chem 160: 37-48 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.073
BindingDB Entry DOI: 10.7270/Q2QV3QXP |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458672

(CHEMBL4203572)Show SMILES [H][C@@]12CCC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1cc(Cl)c(F)cc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C27H25ClF4N2O5/c28-19-13-22-18(12-20(19)29)25(33(15-6-7-15)23(35)10-11-24(36)37)17-2-1-3-21(17)34(22)26(38)14-4-8-16(9-5-14)39-27(30,31)32/h4-5,8-9,12-13,15,17,21,25H,1-3,6-7,10-11H2,(H,36,37)/t17-,21+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458652
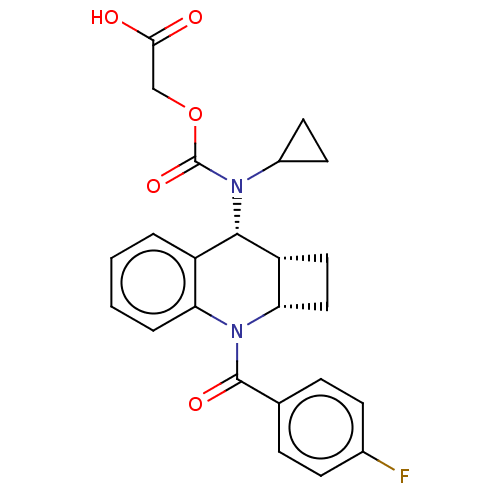
(CHEMBL4202859)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1ccc(F)cc1)c1ccccc1[C@@H]2N(C1CC1)C(=O)OCC(O)=O |r| Show InChI InChI=1S/C24H23FN2O5/c25-15-7-5-14(6-8-15)23(30)27-19-4-2-1-3-17(19)22(18-11-12-20(18)27)26(16-9-10-16)24(31)32-13-21(28)29/h1-8,16,18,20,22H,9-13H2,(H,28,29)/t18-,20+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50520705
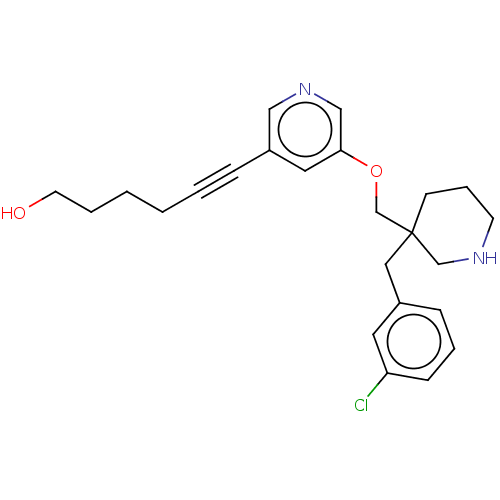
(CHEMBL4458658)Show SMILES OCCCCC#Cc1cncc(OCC2(Cc3cccc(Cl)c3)CCCNC2)c1 Show InChI InChI=1S/C24H29ClN2O2/c25-22-9-5-8-20(13-22)15-24(10-6-11-26-18-24)19-29-23-14-21(16-27-17-23)7-3-1-2-4-12-28/h5,8-9,13-14,16-17,26,28H,1-2,4,6,10-12,15,18-19H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis acetylcholine-binding protein incubated for 60 mins followed by 3 hrs incubation in dark condi... |
Eur J Med Chem 160: 37-48 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.073
BindingDB Entry DOI: 10.7270/Q2QV3QXP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362733
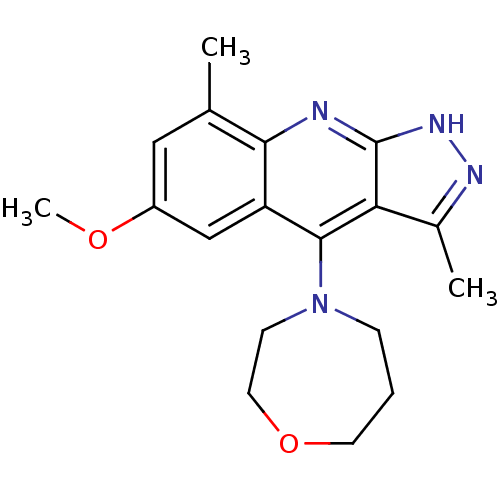
(CHEMBL1939789)Show InChI InChI=1S/C18H22N4O2/c1-11-9-13(23-3)10-14-16(11)19-18-15(12(2)20-21-18)17(14)22-5-4-7-24-8-6-22/h9-10H,4-8H2,1-3H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50380643
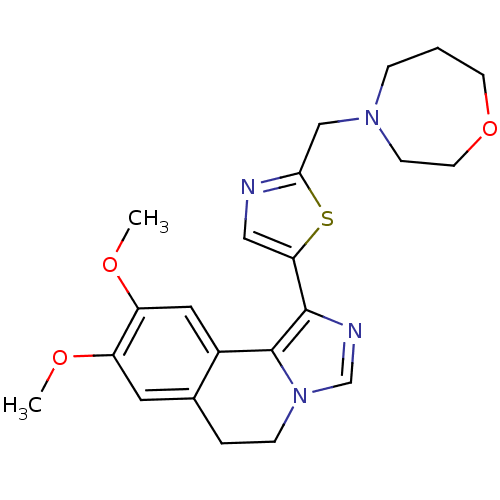
(CHEMBL2017067)Show SMILES COc1cc2CCn3cnc(-c4cnc(CN5CCCOCC5)s4)c3-c2cc1OC Show InChI InChI=1S/C22H26N4O3S/c1-27-17-10-15-4-6-26-14-24-21(22(26)16(15)11-18(17)28-2)19-12-23-20(30-19)13-25-5-3-8-29-9-7-25/h10-12,14H,3-9,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as inhibition of [3H]cAMP hydrolysis by scintillation proximity assay |
Bioorg Med Chem Lett 22: 2585-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.113
BindingDB Entry DOI: 10.7270/Q2319WX5 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458681

(CHEMBL4215576)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1ccccc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C26H25F3N2O5/c27-26(28,29)36-17-9-5-15(6-10-17)25(35)31-20-4-2-1-3-18(20)24(19-11-12-21(19)31)30(16-7-8-16)22(32)13-14-23(33)34/h1-6,9-10,16,19,21,24H,7-8,11-14H2,(H,33,34)/t19-,21+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458677
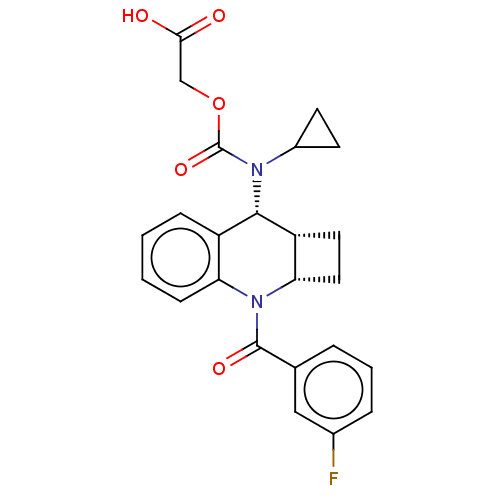
(CHEMBL4212084)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1cccc(F)c1)c1ccccc1[C@@H]2N(C1CC1)C(=O)OCC(O)=O |r| Show InChI InChI=1S/C24H23FN2O5/c25-15-5-3-4-14(12-15)23(30)27-19-7-2-1-6-17(19)22(18-10-11-20(18)27)26(16-8-9-16)24(31)32-13-21(28)29/h1-7,12,16,18,20,22H,8-11,13H2,(H,28,29)/t18-,20+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50380637

(CHEMBL2017061)Show SMILES COc1cc2CCn3cnc(-c4cnc(s4)C(=O)NC4CCOCC4)c3-c2cc1OC Show InChI InChI=1S/C22H24N4O4S/c1-28-16-9-13-3-6-26-12-24-19(20(26)15(13)10-17(16)29-2)18-11-23-22(31-18)21(27)25-14-4-7-30-8-5-14/h9-12,14H,3-8H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as inhibition of [3H]cAMP hydrolysis by scintillation proximity assay |
Bioorg Med Chem Lett 22: 2585-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.113
BindingDB Entry DOI: 10.7270/Q2319WX5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506097
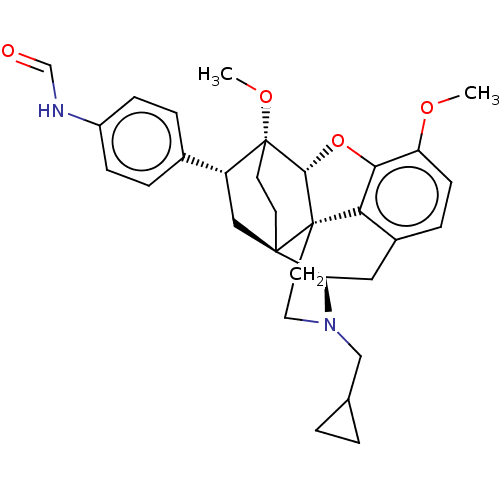
(CHEMBL4546243)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC=O)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C31H36N2O4/c1-35-24-10-7-21-15-25-29-11-12-31(36-2,23(16-29)20-5-8-22(9-6-20)32-18-34)28-30(29,26(21)27(24)37-28)13-14-33(25)17-19-3-4-19/h5-10,18-19,23,25,28H,3-4,11-17H2,1-2H3,(H,32,34)/t23-,25-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data