

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Interleukin-1 beta | ||
| Ligand | BDBM531280 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | IL-1 Secretion Assa | ||
| IC50 | 6.80±n/a nM | ||
| Citation |  Farady, C; Gommermann, N; Janser, P; Mackay, A; Mattes, H; Smith, N; Solovay, CF; Stiefl, NJ; Vangrevelinghe, E; Velcicky, J; von Matt, A NLRP3 inflammasome inhibitors US Patent US11208399 Publication Date 12/28/2021 Farady, C; Gommermann, N; Janser, P; Mackay, A; Mattes, H; Smith, N; Solovay, CF; Stiefl, NJ; Vangrevelinghe, E; Velcicky, J; von Matt, A NLRP3 inflammasome inhibitors US Patent US11208399 Publication Date 12/28/2021 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Interleukin-1 beta | |||
| Name: | Interleukin-1 beta | ||
| Synonyms: | IL1B | IL1B_HUMAN | IL1F2 | Interleukin 1-beta | Interleukin-1 beta | hIL-1beta | ||
| Type: | Cytokine | ||
| Mol. Mass.: | 30734.47 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P01584 | ||
| Residue: | 269 | ||
| Sequence: |
| ||
| BDBM531280 | |||
| n/a | |||
| Name | BDBM531280 | ||
| Synonyms: | US11208399, Example 40 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H25F3N4O | ||
| Mol. Mass. | 394.4339 | ||
| SMILES | CN1CCC[C@H](C1)Nc1nnc(c(C)c1C)-c1c(C)cc(cc1O)C(F)(F)F |r,wD:5.7,(8,-3.36,;6.67,-2.59,;6.67,-1.05,;5.33,-.28,;4,-1.05,;4,-2.59,;5.33,-3.36,;2.67,-3.36,;1.33,-2.59,;,-3.36,;-1.33,-2.59,;-1.33,-1.05,;,-.28,;,1.26,;1.33,-1.05,;2.67,-.28,;-2.67,-.28,;-2.67,1.26,;-1.33,2.03,;-4,2.03,;-5.38,1.29,;-5.33,-.28,;-4,-1.05,;-4,-2.59,;-6.72,2.06,;-8.05,2.83,;-5.95,3.39,;-7.49,.72,)| | ||
| Structure | 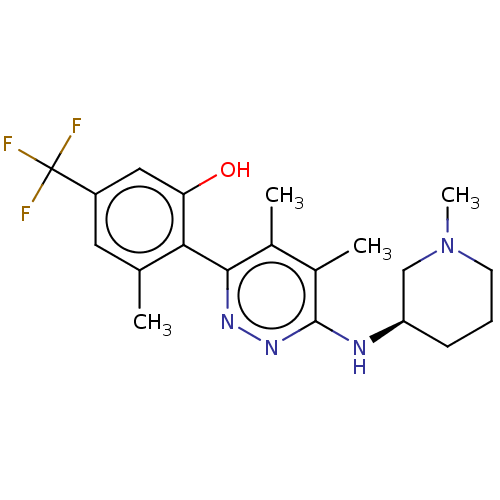
| ||