| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2A6 |
|---|
| Ligand | BDBM21358 |
|---|
| Substrate/Competitor | BDBM12342 |
|---|
| Meas. Tech. | Cytochrome P450 Inhibition |
|---|
| pH | 7.4±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| IC50 | 41000±n/a nM |
|---|
| Citation |  Cole, DC; Stock, JR; Lennox, WJ; Bernotas, RC; Ellingboe, JW; Boikess, S; Coupet, J; Smith, DL; Leung, L; Zhang, GM; Feng, X; Kelly, MF; Galante, R; Huang, P; Dawson, LA; Marquis, K; Rosenzweig-Lipson, S; Beyer, CE; Schechter, LE Discovery of N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine as a potent, selective, and orally active 5-HT(6) receptor agonist. J Med Chem50:5535-8 (2007) [PubMed] Article Cole, DC; Stock, JR; Lennox, WJ; Bernotas, RC; Ellingboe, JW; Boikess, S; Coupet, J; Smith, DL; Leung, L; Zhang, GM; Feng, X; Kelly, MF; Galante, R; Huang, P; Dawson, LA; Marquis, K; Rosenzweig-Lipson, S; Beyer, CE; Schechter, LE Discovery of N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine as a potent, selective, and orally active 5-HT(6) receptor agonist. J Med Chem50:5535-8 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Cytochrome P450 2A6 |
|---|
| Name: | Cytochrome P450 2A6 |
|---|
| Synonyms: | 1,4-cineole 2-exo-monooxygenase | 1.14.13.- | CP2A6_HUMAN | CYP2A3 | CYP2A6 | CYPIIA6 | Coumarin 7-hydroxylase | Cytochrome P450 2A6 | Cytochrome P450 IIA3 | Cytochrome P450(I) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56514.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11509 |
|---|
| Residue: | 494 |
|---|
| Sequence: | MLASGMLLVALLVCLTVMVLMSVWQQRKSKGKLPPGPTPLPFIGNYLQLNTEQMYNSLMK
ISERYGPVFTIHLGPRRVVVLCGHDAVREALVDQAEEFSGRGEQATFDWVFKGYGVVFSN
GERAKQLRRFSIATLRDFGVGKRGIEERIQEEAGFLIDALRGTGGANIDPTFFLSRTVSN
VISSIVFGDRFDYKDKEFLSLLRMMLGIFQFTSTSTGQLYEMFSSVMKHLPGPQQQAFQL
LQGLEDFIAKKVEHNQRTLDPNSPRDFIDSFLIRMQEEEKNPNTEFYLKNLVMTTLNLFI
GGTETVSTTLRYGFLLLMKHPEVEAKVHEEIDRVIGKNRQPKFEDRAKMPYMEAVIHEIQ
RFGDVIPMSLARRVKKDTKFRDFFLPKGTEVYPMLGSVLRDPSFFSNPQDFNPQHFLNEK
GQFKKSDAFVPFSIGKRNCFGEGLARMELFLFFTTVMQNFRLKSSQSPKDIDVSPKHVGF
ATIPRNYTMSFLPR
|
|
|
|---|
| BDBM21358 |
|---|
| BDBM12342 |
|---|
| Name | BDBM21358 |
|---|
| Synonyms: | 2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulfonyl)-1H-indol-3-yl]ethan-1-amine | N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine | SAX-187 | WAY-181187 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H13ClN4O2S2 |
|---|
| Mol. Mass. | 380.872 |
|---|
| SMILES | NCCc1cn(c2ccccc12)S(=O)(=O)c1c(Cl)nc2sccn12 |
|---|
| Structure | 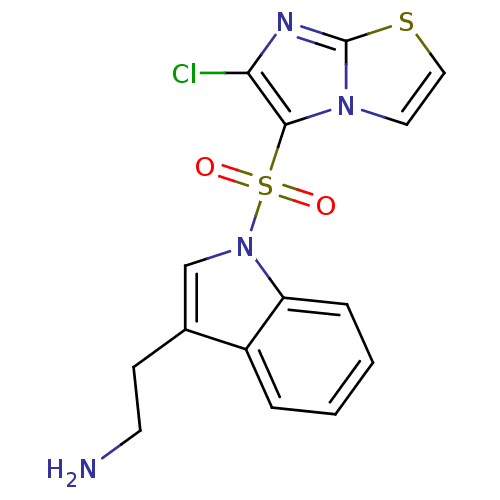
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cole, DC; Stock, JR; Lennox, WJ; Bernotas, RC; Ellingboe, JW; Boikess, S; Coupet, J; Smith, DL; Leung, L; Zhang, GM; Feng, X; Kelly, MF; Galante, R; Huang, P; Dawson, LA; Marquis, K; Rosenzweig-Lipson, S; Beyer, CE; Schechter, LE Discovery of N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine as a potent, selective, and orally active 5-HT(6) receptor agonist. J Med Chem50:5535-8 (2007) [PubMed] Article
Cole, DC; Stock, JR; Lennox, WJ; Bernotas, RC; Ellingboe, JW; Boikess, S; Coupet, J; Smith, DL; Leung, L; Zhang, GM; Feng, X; Kelly, MF; Galante, R; Huang, P; Dawson, LA; Marquis, K; Rosenzweig-Lipson, S; Beyer, CE; Schechter, LE Discovery of N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine as a potent, selective, and orally active 5-HT(6) receptor agonist. J Med Chem50:5535-8 (2007) [PubMed] Article