 Found 1928 hits with Last Name = 'leung' and Initial = 'l'
Found 1928 hits with Last Name = 'leung' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410903
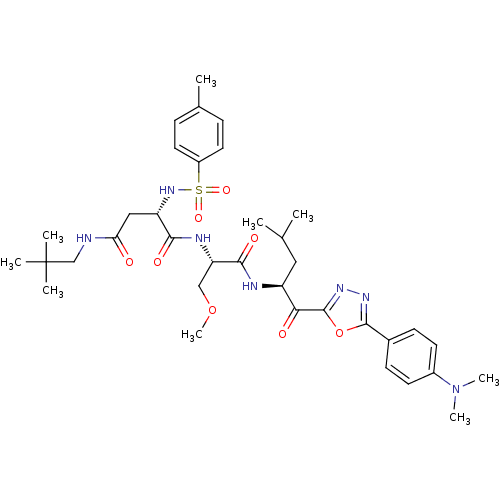
(CHEMBL207598)Show SMILES COC[C@H](NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc(o1)-c1ccc(cc1)N(C)C Show InChI InChI=1S/C36H51N7O8S/c1-22(2)18-27(31(45)35-41-40-34(51-35)24-12-14-25(15-13-24)43(7)8)38-33(47)29(20-50-9)39-32(46)28(19-30(44)37-21-36(4,5)6)42-52(48,49)26-16-10-23(3)11-17-26/h10-17,22,27-29,42H,18-21H2,1-9H3,(H,37,44)(H,38,47)(H,39,46)/t27-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410898
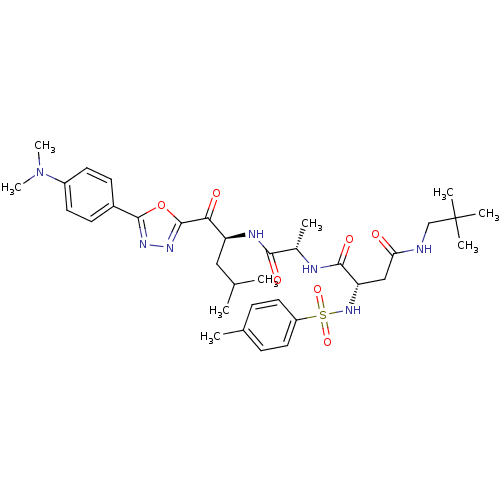
(CHEMBL205757)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)c1nnc(o1)-c1ccc(cc1)N(C)C Show InChI InChI=1S/C35H49N7O7S/c1-21(2)18-27(30(44)34-40-39-33(49-34)24-12-14-25(15-13-24)42(8)9)38-31(45)23(4)37-32(46)28(19-29(43)36-20-35(5,6)7)41-50(47,48)26-16-10-22(3)11-17-26/h10-17,21,23,27-28,41H,18-20H2,1-9H3,(H,36,43)(H,37,46)(H,38,45)/t23-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.545 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410901
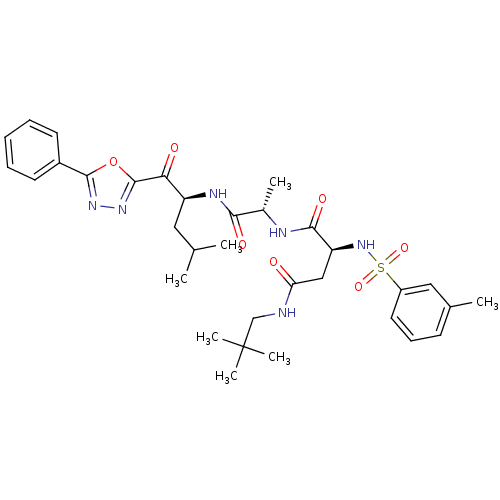
(CHEMBL206413)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1cccc(C)c1)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C33H44N6O7S/c1-20(2)16-25(28(41)32-38-37-31(46-32)23-13-9-8-10-14-23)36-29(42)22(4)35-30(43)26(18-27(40)34-19-33(5,6)7)39-47(44,45)24-15-11-12-21(3)17-24/h8-15,17,20,22,25-26,39H,16,18-19H2,1-7H3,(H,34,40)(H,35,43)(H,36,42)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.679 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219599
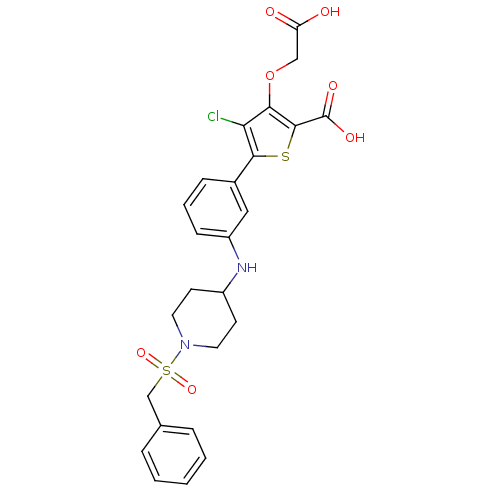
(5-(3-{[1-(benzylsulfonyl)piperidin-4-yl]amino}phen...)Show SMILES OC(=O)COc1c(Cl)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25ClN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410899

(CHEMBL383674)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C33H44N6O7S/c1-20(2)17-25(28(41)32-38-37-31(46-32)23-11-9-8-10-12-23)36-29(42)22(4)35-30(43)26(18-27(40)34-19-33(5,6)7)39-47(44,45)24-15-13-21(3)14-16-24/h8-16,20,22,25-26,39H,17-19H2,1-7H3,(H,34,40)(H,35,43)(H,36,42)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410904
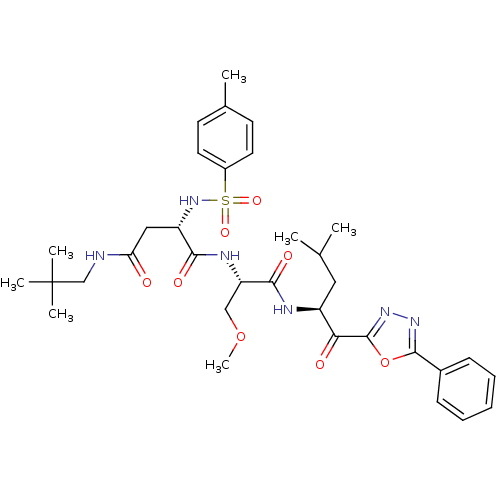
(CHEMBL377532)Show SMILES COC[C@H](NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H46N6O8S/c1-21(2)17-25(29(42)33-39-38-32(48-33)23-11-9-8-10-12-23)36-31(44)27(19-47-7)37-30(43)26(18-28(41)35-20-34(4,5)6)40-49(45,46)24-15-13-22(3)14-16-24/h8-16,21,25-27,40H,17-20H2,1-7H3,(H,35,41)(H,36,44)(H,37,43)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219588
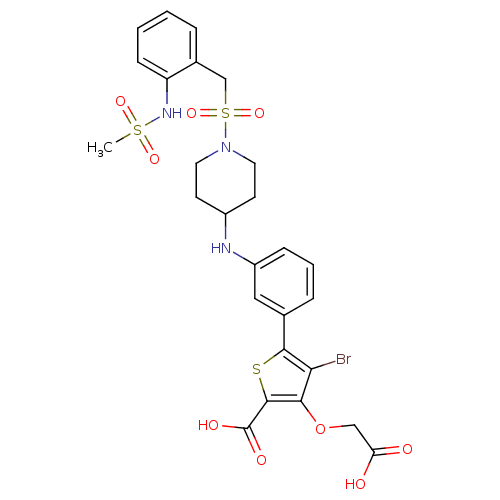
(4-bromo-3-(carboxymethoxy)-5-(3-{[1-({2-[(methylsu...)Show SMILES CS(=O)(=O)Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C26H28BrN3O9S3/c1-41(35,36)29-20-8-3-2-5-17(20)15-42(37,38)30-11-9-18(10-12-30)28-19-7-4-6-16(13-19)24-22(27)23(39-14-21(31)32)25(40-24)26(33)34/h2-8,13,18,28-29H,9-12,14-15H2,1H3,(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21351
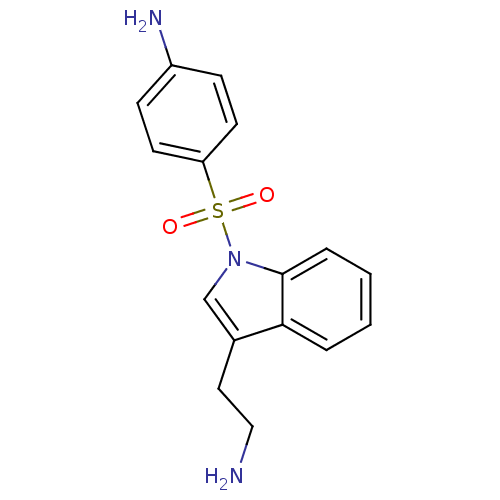
(4-{[3-(2-aminoethyl)-1H-indole-1-]sulfonyl}aniline...)Show InChI InChI=1S/C16H17N3O2S/c17-10-9-12-11-19(16-4-2-1-3-15(12)16)22(20,21)14-7-5-13(18)6-8-14/h1-8,11H,9-10,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | 91 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219584
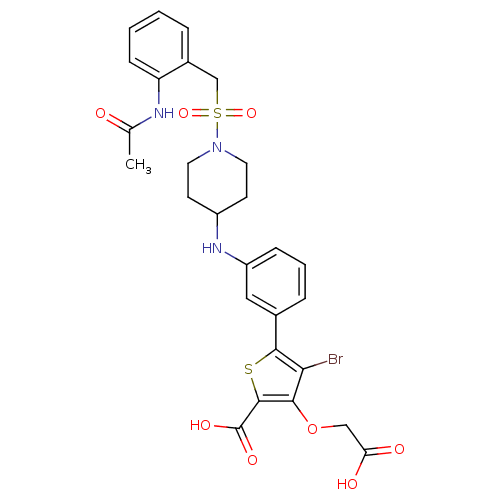
(5-{3-[(1-{[2-(acetylamino)benzyl]sulfonyl}piperidi...)Show SMILES CC(=O)Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C27H28BrN3O8S2/c1-16(32)29-21-8-3-2-5-18(21)15-41(37,38)31-11-9-19(10-12-31)30-20-7-4-6-17(13-20)25-23(28)24(39-14-22(33)34)26(40-25)27(35)36/h2-8,13,19,30H,9-12,14-15H2,1H3,(H,29,32)(H,33,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219577
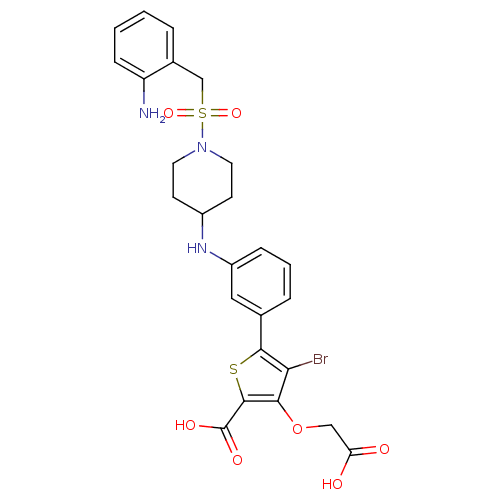
(5-[3-({1-[(2-aminobenzyl)sulfonyl]piperidin-4-yl}a...)Show SMILES Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C25H26BrN3O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)15-5-3-6-18(12-15)28-17-8-10-29(11-9-17)38(34,35)14-16-4-1-2-7-19(16)27/h1-7,12,17,28H,8-11,13-14,27H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219586
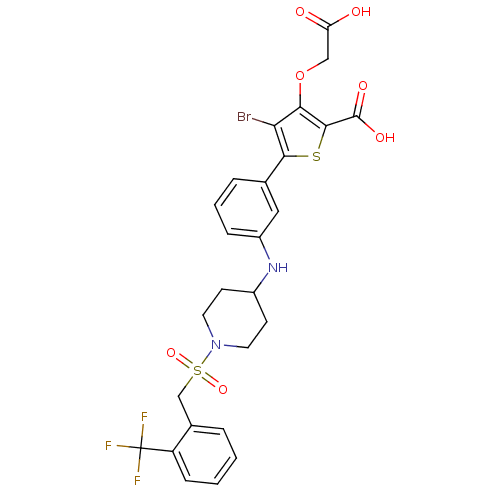
(4-bromo-3-(carboxymethoxy)-5-{3-[(1-{[2-(trifluoro...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2C(F)(F)F)c1 Show InChI InChI=1S/C26H24BrF3N2O7S2/c27-21-22(39-13-20(33)34)24(25(35)36)40-23(21)15-5-3-6-18(12-15)31-17-8-10-32(11-9-17)41(37,38)14-16-4-1-2-7-19(16)26(28,29)30/h1-7,12,17,31H,8-11,13-14H2,(H,33,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21358
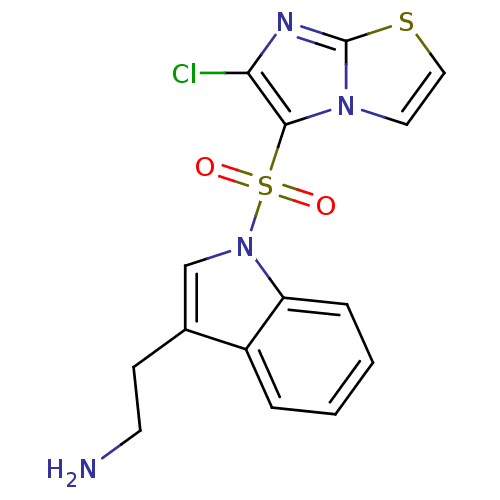
(2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...)Show SMILES NCCc1cn(c2ccccc12)S(=O)(=O)c1c(Cl)nc2sccn12 Show InChI InChI=1S/C15H13ClN4O2S2/c16-13-14(19-7-8-23-15(19)18-13)24(21,22)20-9-10(5-6-17)11-3-1-2-4-12(11)20/h1-4,7-9H,5-6,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | -49.2 | n/a | n/a | 6.5 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219577

(5-[3-({1-[(2-aminobenzyl)sulfonyl]piperidin-4-yl}a...)Show SMILES Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C25H26BrN3O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)15-5-3-6-18(12-15)28-17-8-10-29(11-9-17)38(34,35)14-16-4-1-2-7-19(16)27/h1-7,12,17,28H,8-11,13-14,27H2,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219584

(5-{3-[(1-{[2-(acetylamino)benzyl]sulfonyl}piperidi...)Show SMILES CC(=O)Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C27H28BrN3O8S2/c1-16(32)29-21-8-3-2-5-18(21)15-41(37,38)31-11-9-19(10-12-31)30-20-7-4-6-17(13-20)25-23(28)24(39-14-22(33)34)26(40-25)27(35)36/h2-8,13,19,30H,9-12,14-15H2,1H3,(H,29,32)(H,33,34)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219588

(4-bromo-3-(carboxymethoxy)-5-(3-{[1-({2-[(methylsu...)Show SMILES CS(=O)(=O)Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C26H28BrN3O9S3/c1-41(35,36)29-20-8-3-2-5-17(20)15-42(37,38)30-11-9-18(10-12-30)28-19-7-4-6-16(13-19)24-22(27)23(39-14-21(31)32)25(40-24)26(33)34/h2-8,13,18,28-29H,9-12,14-15H2,1H3,(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069984

((R)-1-((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)B(O)O Show InChI InChI=1S/C25H42BN3O6/c1-16(2)12-20(24(31)29-22(26(33)34)14-18(5)6)27-23(30)21(13-17(3)4)28-25(32)35-15-19-10-8-7-9-11-19/h7-11,16-18,20-22,33-34H,12-15H2,1-6H3,(H,27,30)(H,28,32)(H,29,31)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219569
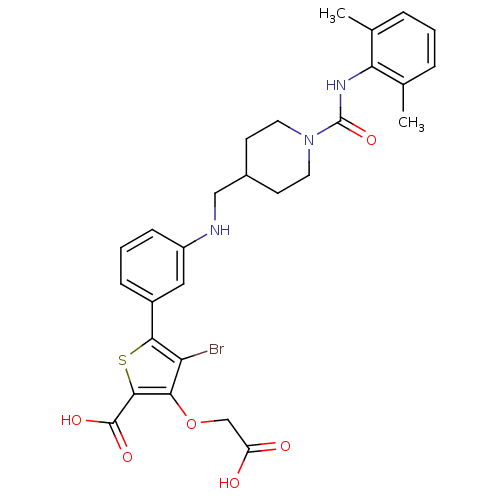
(4-bromo-3-carboxymethoxy-5-(3-{[1-(2,6-dimethylphe...)Show SMILES Cc1cccc(C)c1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C28H30BrN3O6S/c1-16-5-3-6-17(2)23(16)31-28(37)32-11-9-18(10-12-32)14-30-20-8-4-7-19(13-20)25-22(29)24(38-15-21(33)34)26(39-25)27(35)36/h3-8,13,18,30H,9-12,14-15H2,1-2H3,(H,31,37)(H,33,34)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219570
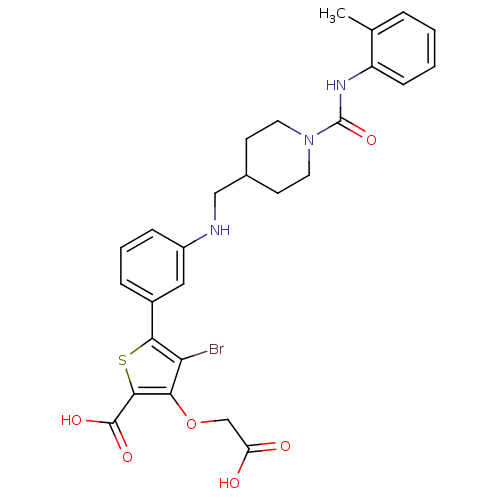
(4-bromo-3-carboxymethoxy-5-{3-[(1-o-tolylcarbamoyl...)Show SMILES Cc1ccccc1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C27H28BrN3O6S/c1-16-5-2-3-8-20(16)30-27(36)31-11-9-17(10-12-31)14-29-19-7-4-6-18(13-19)24-22(28)23(37-15-21(32)33)25(38-24)26(34)35/h2-8,13,17,29H,9-12,14-15H2,1H3,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219585
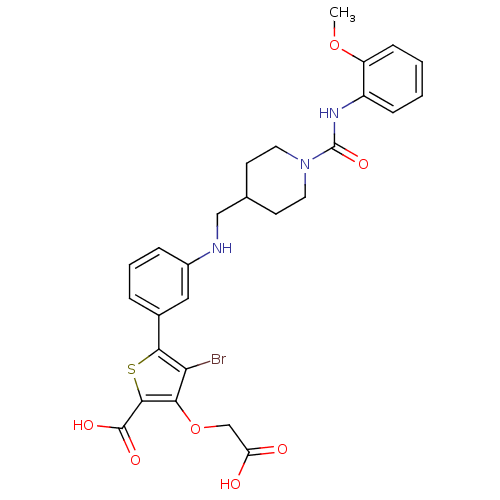
(4-bromo-3-carboxymethoxy-5-(3-{[1-(2-methoxyphenyl...)Show SMILES COc1ccccc1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C27H28BrN3O7S/c1-37-20-8-3-2-7-19(20)30-27(36)31-11-9-16(10-12-31)14-29-18-6-4-5-17(13-18)24-22(28)23(38-15-21(32)33)25(39-24)26(34)35/h2-8,13,16,29H,9-12,14-15H2,1H3,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069985
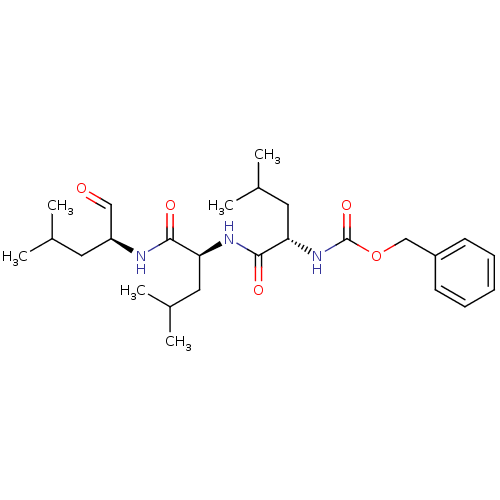
((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219567
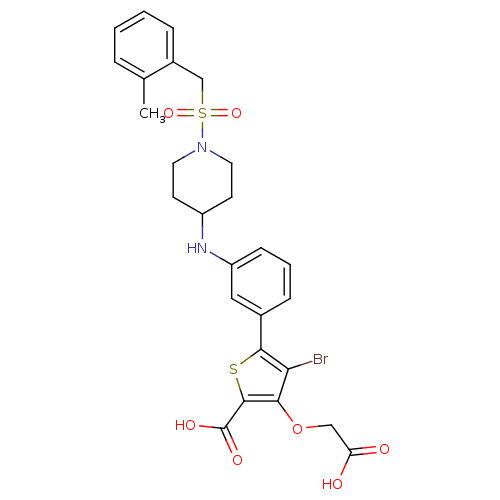
(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(2-methylbenz...)Show SMILES Cc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C26H27BrN2O7S2/c1-16-5-2-3-6-18(16)15-38(34,35)29-11-9-19(10-12-29)28-20-8-4-7-17(13-20)24-22(27)23(36-14-21(30)31)25(37-24)26(32)33/h2-8,13,19,28H,9-12,14-15H2,1H3,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219568

(4-bromo-3-carboxymethoxy-5-(3-{[1-(2-chlorophenylc...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NCC2CCN(CC2)C(=O)Nc2ccccc2Cl)c1 Show InChI InChI=1S/C26H25BrClN3O6S/c27-21-22(37-14-20(32)33)24(25(34)35)38-23(21)16-4-3-5-17(12-16)29-13-15-8-10-31(11-9-15)26(36)30-19-7-2-1-6-18(19)28/h1-7,12,15,29H,8-11,13-14H2,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219566
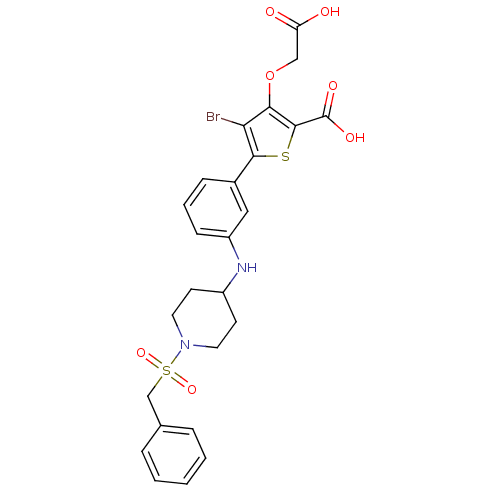
(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219575
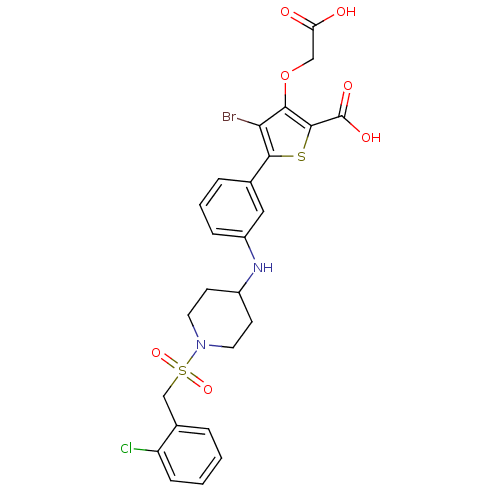
(3-carboxymethoxy-5-{3-[1-(2-chlorobenzenesulfonyl)...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2Cl)c1 Show InChI InChI=1S/C25H24BrClN2O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)15-5-3-6-18(12-15)28-17-8-10-29(11-9-17)38(34,35)14-16-4-1-2-7-19(16)27/h1-7,12,17,28H,8-11,13-14H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219566

(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219575

(3-carboxymethoxy-5-{3-[1-(2-chlorobenzenesulfonyl)...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2Cl)c1 Show InChI InChI=1S/C25H24BrClN2O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)15-5-3-6-18(12-15)28-17-8-10-29(11-9-17)38(34,35)14-16-4-1-2-7-19(16)27/h1-7,12,17,28H,8-11,13-14H2,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219570

(4-bromo-3-carboxymethoxy-5-{3-[(1-o-tolylcarbamoyl...)Show SMILES Cc1ccccc1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C27H28BrN3O6S/c1-16-5-2-3-8-20(16)30-27(36)31-11-9-17(10-12-31)14-29-19-7-4-6-18(13-19)24-22(28)23(37-15-21(32)33)25(38-24)26(34)35/h2-8,13,17,29H,9-12,14-15H2,1H3,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219585

(4-bromo-3-carboxymethoxy-5-(3-{[1-(2-methoxyphenyl...)Show SMILES COc1ccccc1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C27H28BrN3O7S/c1-37-20-8-3-2-7-19(20)30-27(36)31-11-9-16(10-12-31)14-29-18-6-4-5-17(13-18)24-22(28)23(38-15-21(32)33)25(39-24)26(34)35/h2-8,13,16,29H,9-12,14-15H2,1H3,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410906

(CHEMBL207160)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(C)(=O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C27H40N6O7S/c1-16(2)13-19(22(35)26-32-31-25(40-26)18-11-9-8-10-12-18)30-23(36)17(3)29-24(37)20(33-41(7,38)39)14-21(34)28-15-27(4,5)6/h8-12,16-17,19-20,33H,13-15H2,1-7H3,(H,28,34)(H,29,37)(H,30,36)/t17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219586

(4-bromo-3-(carboxymethoxy)-5-{3-[(1-{[2-(trifluoro...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2C(F)(F)F)c1 Show InChI InChI=1S/C26H24BrF3N2O7S2/c27-21-22(39-13-20(33)34)24(25(35)36)40-23(21)15-5-3-6-18(12-15)31-17-8-10-32(11-9-17)41(37,38)14-16-4-1-2-7-19(16)26(28,29)30/h1-7,12,17,31H,8-11,13-14H2,(H,33,34)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21345
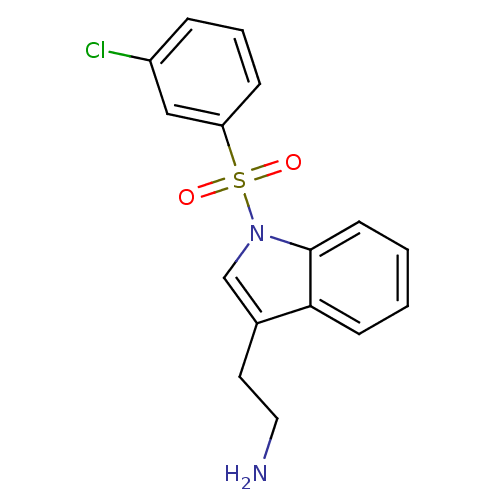
(2-{1-[(3-chlorobenzene)sulfonyl]-1H-indol-3-yl}eth...)Show InChI InChI=1S/C16H15ClN2O2S/c17-13-4-3-5-14(10-13)22(20,21)19-11-12(8-9-18)15-6-1-2-7-16(15)19/h1-7,10-11H,8-9,18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -46.5 | n/a | n/a | 109 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219568

(4-bromo-3-carboxymethoxy-5-(3-{[1-(2-chlorophenylc...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NCC2CCN(CC2)C(=O)Nc2ccccc2Cl)c1 Show InChI InChI=1S/C26H25BrClN3O6S/c27-21-22(37-14-20(32)33)24(25(34)35)38-23(21)16-4-3-5-17(12-16)29-13-15-8-10-31(11-9-15)26(36)30-19-7-2-1-6-18(19)28/h1-7,12,15,29H,8-11,13-14H2,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219567

(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(2-methylbenz...)Show SMILES Cc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C26H27BrN2O7S2/c1-16-5-2-3-6-18(16)15-38(34,35)29-11-9-19(10-12-29)28-20-8-4-7-17(13-20)24-22(27)23(36-14-21(30)31)25(37-24)26(32)33/h2-8,13,19,28H,9-12,14-15H2,1H3,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21354
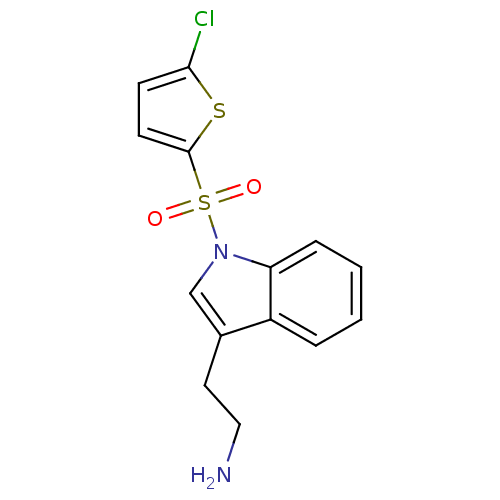
(2-{1-[(5-chlorothiophene-2-)sulfonyl]-1H-indol-3-y...)Show InChI InChI=1S/C14H13ClN2O2S2/c15-13-5-6-14(20-13)21(18,19)17-9-10(7-8-16)11-3-1-2-4-12(11)17/h1-6,9H,7-8,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -46.5 | n/a | n/a | 181 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219565
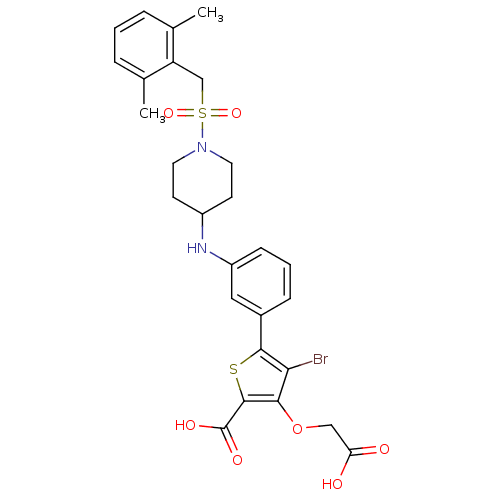
(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(2,6-dimethyl...)Show SMILES Cc1cccc(C)c1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C27H29BrN2O7S2/c1-16-5-3-6-17(2)21(16)15-39(35,36)30-11-9-19(10-12-30)29-20-8-4-7-18(13-20)25-23(28)24(37-14-22(31)32)26(38-25)27(33)34/h3-8,13,19,29H,9-12,14-15H2,1-2H3,(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21353
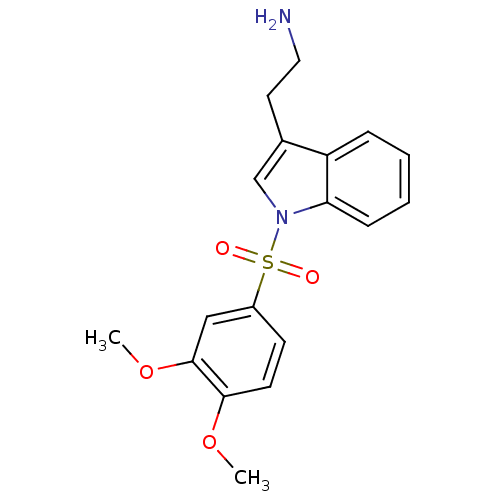
(2-{1-[(3,4-dimethoxybenzene)sulfonyl]-1H-indol-3-y...)Show InChI InChI=1S/C18H20N2O4S/c1-23-17-8-7-14(11-18(17)24-2)25(21,22)20-12-13(9-10-19)15-5-3-4-6-16(15)20/h3-8,11-12H,9-10,19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.1 | n/a | n/a | 17.5 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219591
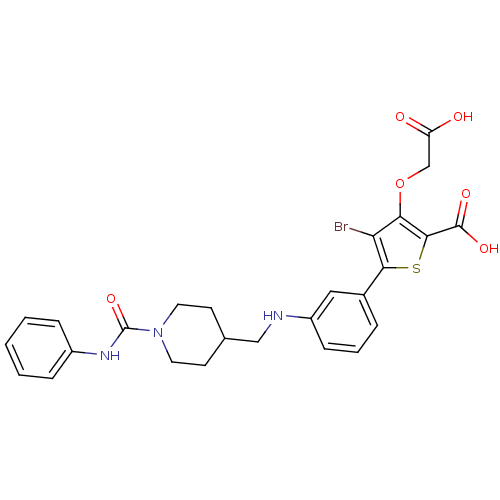
(5-[3-({[1-(anilinocarbonyl)piperidin-4-yl]methyl}a...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NCC2CCN(CC2)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C26H26BrN3O6S/c27-21-22(36-15-20(31)32)24(25(33)34)37-23(21)17-5-4-8-19(13-17)28-14-16-9-11-30(12-10-16)26(35)29-18-6-2-1-3-7-18/h1-8,13,16,28H,9-12,14-15H2,(H,29,35)(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21355
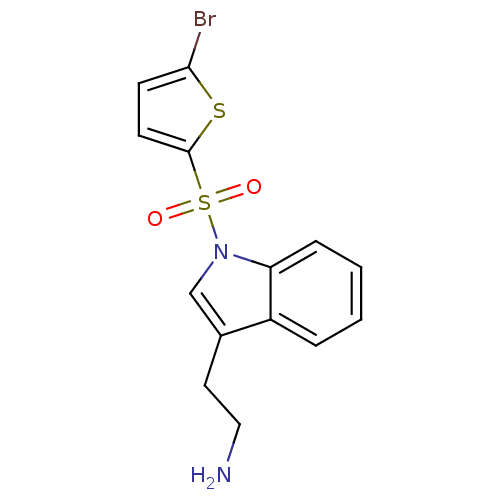
(2-{1-[(5-bromothiophene-2-)sulfonyl]-1H-indol-3-yl...)Show InChI InChI=1S/C14H13BrN2O2S2/c15-13-5-6-14(20-13)21(18,19)17-9-10(7-8-16)11-3-1-2-4-12(11)17/h1-6,9H,7-8,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.1 | n/a | n/a | 54 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21349
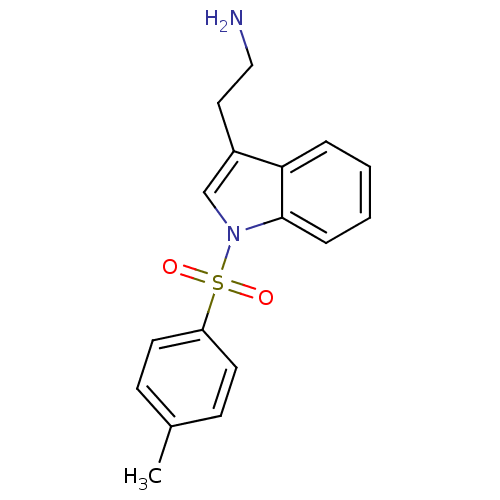
(2-{1-[(4-methylbenzene)sulfonyl]-1H-indol-3-yl}eth...)Show InChI InChI=1S/C17H18N2O2S/c1-13-6-8-15(9-7-13)22(20,21)19-12-14(10-11-18)16-4-2-3-5-17(16)19/h2-9,12H,10-11,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -45.7 | n/a | n/a | 191 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219589
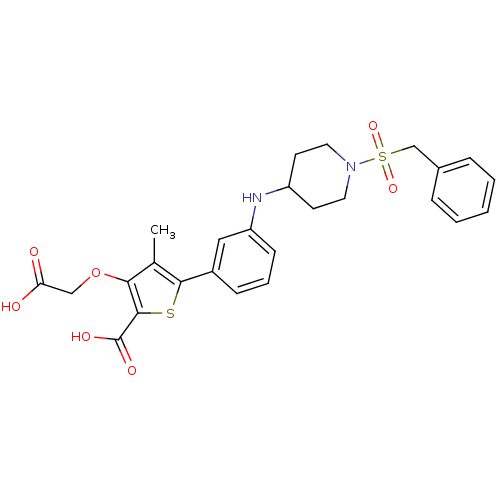
(5-(3-{[1-(benzylsulfonyl)piperidin-4-yl]amino}phen...)Show SMILES Cc1c(OCC(O)=O)c(sc1-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1)C(O)=O Show InChI InChI=1S/C26H28N2O7S2/c1-17-23(35-15-22(29)30)25(26(31)32)36-24(17)19-8-5-9-21(14-19)27-20-10-12-28(13-11-20)37(33,34)16-18-6-3-2-4-7-18/h2-9,14,20,27H,10-13,15-16H2,1H3,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219565

(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(2,6-dimethyl...)Show SMILES Cc1cccc(C)c1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C27H29BrN2O7S2/c1-16-5-3-6-17(2)21(16)15-39(35,36)30-11-9-19(10-12-30)29-20-8-4-7-18(13-20)25-23(28)24(37-14-22(31)32)26(38-25)27(33)34/h3-8,13,19,29H,9-12,14-15H2,1-2H3,(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21365
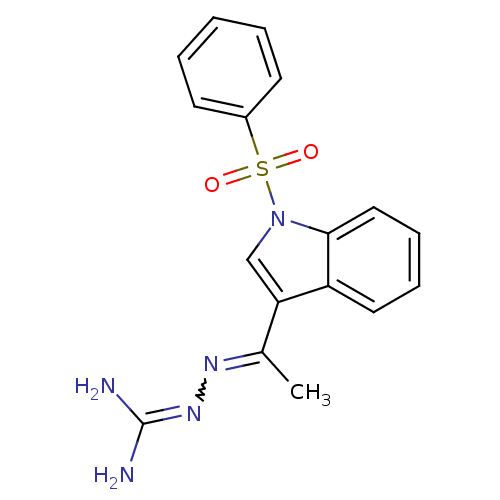
(3-[(E)-{1-[1-(benzenesulfonyl)-1H-indol-3-yl]ethyl...)Show SMILES [#6]-[#6](=[#7]\[#7]=[#6](/[#7])-[#7])-c1cn(c2ccccc12)S(=O)(=O)c1ccccc1 |w:2.2| Show InChI InChI=1S/C17H17N5O2S/c1-12(20-21-17(18)19)15-11-22(16-10-6-5-9-14(15)16)25(23,24)13-7-3-2-4-8-13/h2-11H,1H3,(H4,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219576
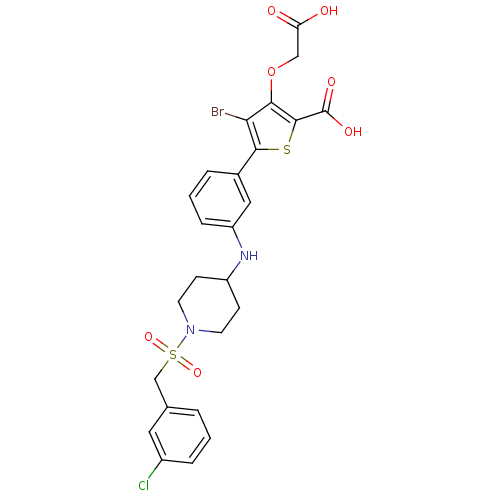
(4-bromo-3-(carboxymethoxy)-5-(3-(1-(3-chlorobenzyl...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2cccc(Cl)c2)c1 Show InChI InChI=1S/C25H24BrClN2O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)16-4-2-6-19(12-16)28-18-7-9-29(10-8-18)38(34,35)14-15-3-1-5-17(27)11-15/h1-6,11-12,18,28H,7-10,13-14H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219569

(4-bromo-3-carboxymethoxy-5-(3-{[1-(2,6-dimethylphe...)Show SMILES Cc1cccc(C)c1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C28H30BrN3O6S/c1-16-5-3-6-17(2)23(16)31-28(37)32-11-9-18(10-12-32)14-30-20-8-4-7-19(13-20)25-22(29)24(38-15-21(33)34)26(39-25)27(35)36/h3-8,13,18,30H,9-12,14-15H2,1-2H3,(H,31,37)(H,33,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM21357
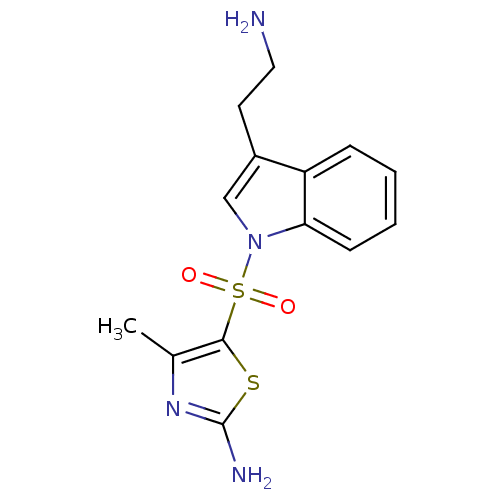
(5-thiazolylsulfonyl tryptamine, 11p | 5-{[3-(2-ami...)Show InChI InChI=1S/C14H16N4O2S2/c1-9-13(21-14(16)17-9)22(19,20)18-8-10(6-7-15)11-4-2-3-5-12(11)18/h2-5,8H,6-7,15H2,1H3,(H2,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -45.2 | n/a | n/a | 9.60 | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... |
J Med Chem 50: 5535-8 (2007)
Article DOI: 10.1021/jm070521y
BindingDB Entry DOI: 10.7270/Q23R0R5G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data