

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114327 BindingDB Entry DOI: 10.7270/Q2NP28GR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21351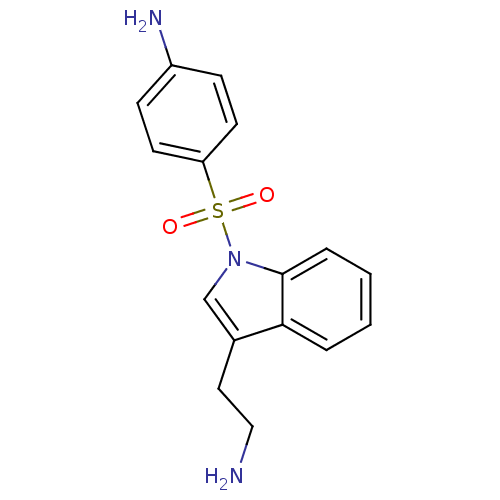 (4-{[3-(2-aminoethyl)-1H-indole-1-]sulfonyl}aniline...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | 91 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50035738 ((R)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to dopamine transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.2 | n/a | n/a | 6.5 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125333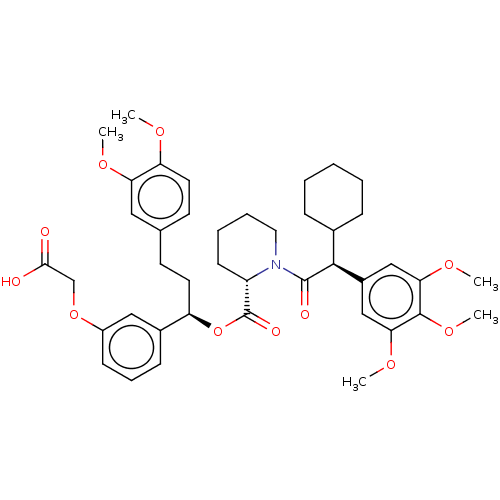 (CHEMBL3623612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125333 (CHEMBL3623612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21354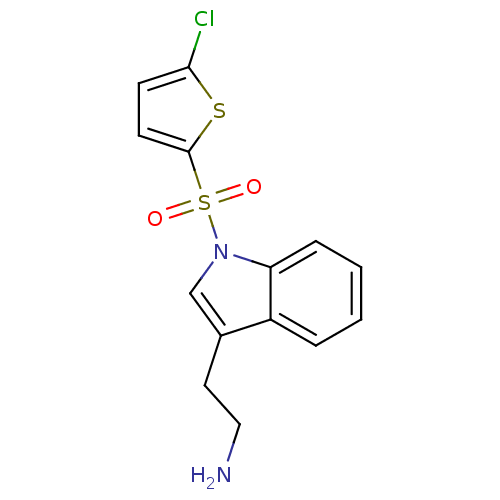 (2-{1-[(5-chlorothiophene-2-)sulfonyl]-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | 181 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125330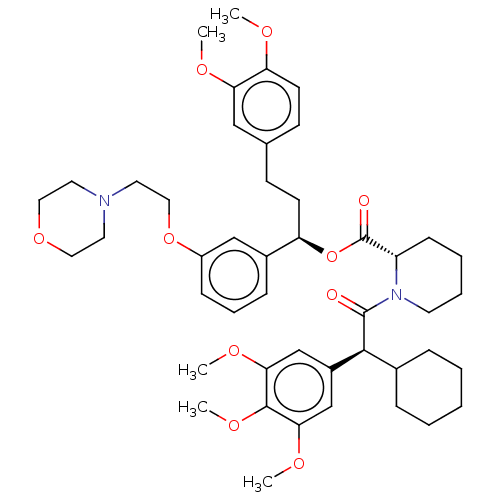 (CHEMBL3623630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125330 (CHEMBL3623630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21345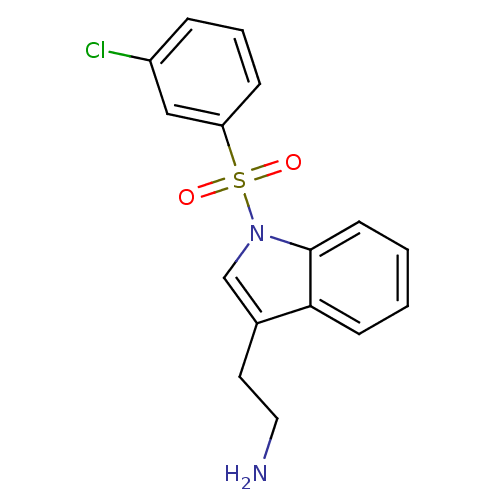 (2-{1-[(3-chlorobenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | 109 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50129369 (3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]o...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to dopamine transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21355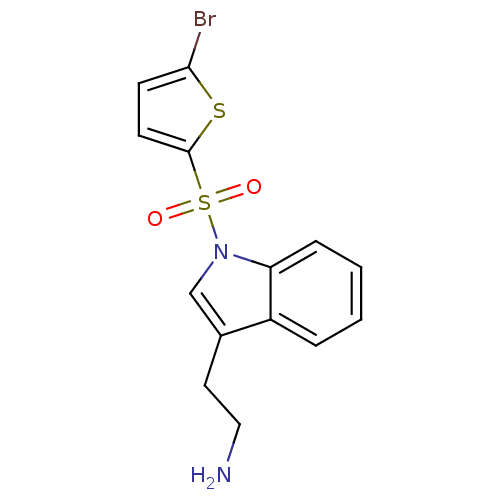 (2-{1-[(5-bromothiophene-2-)sulfonyl]-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.1 | n/a | n/a | 54 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21353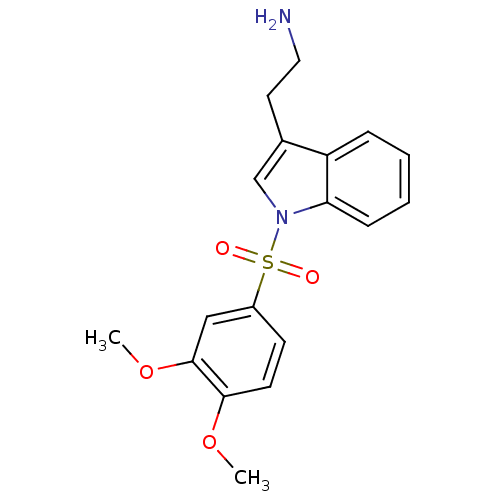 (2-{1-[(3,4-dimethoxybenzene)sulfonyl]-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.1 | n/a | n/a | 17.5 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50129369 (3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]o...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to Serotonin transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21349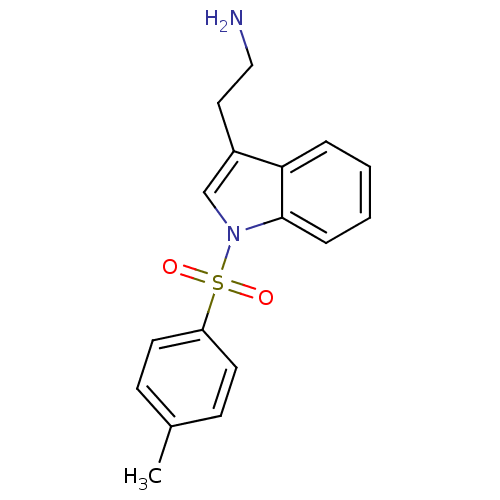 (2-{1-[(4-methylbenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | 191 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21365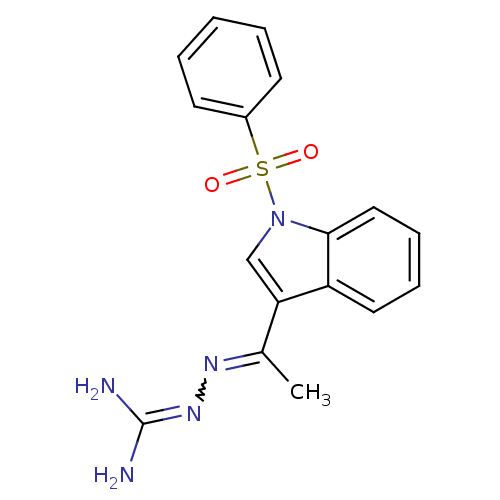 (3-[(E)-{1-[1-(benzenesulfonyl)-1H-indol-3-yl]ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21347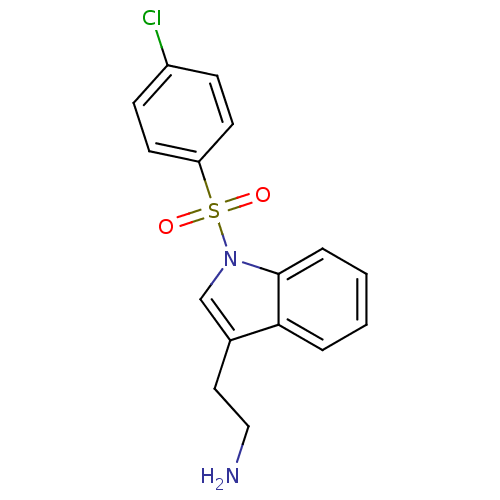 (2-{1-[(4-chlorobenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 174 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21341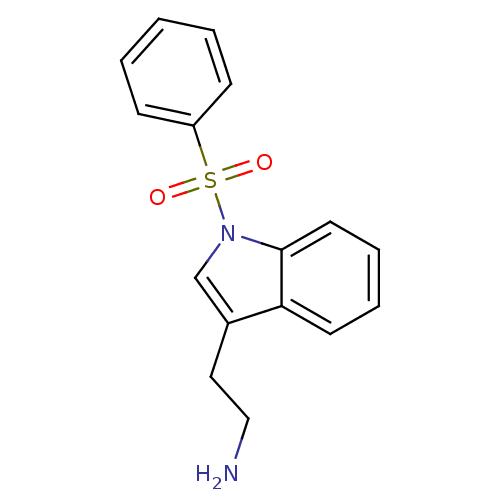 (2-[1-(benzenesulfonyl)-1H-indol-3-yl]ethan-1-amine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 73 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21357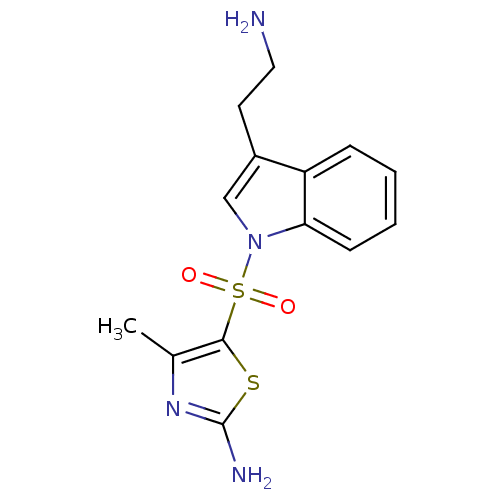 (5-thiazolylsulfonyl tryptamine, 11p | 5-{[3-(2-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 9.60 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21366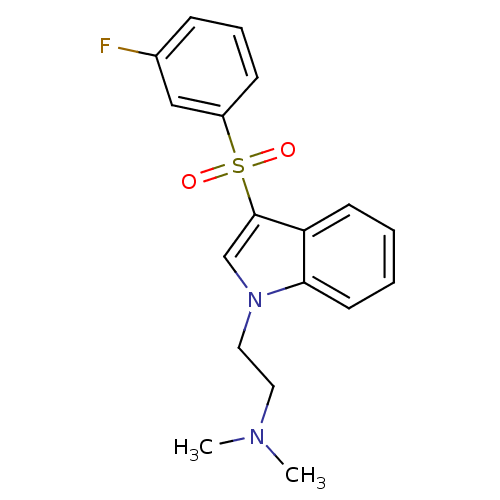 ((2-{3-[(3-fluorobenzene)sulfonyl]-1H-indol-1-yl}et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | -45.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21343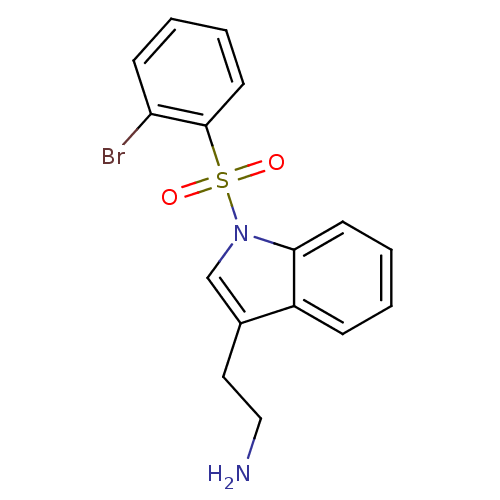 (2-{1-[(2-bromobenzene)sulfonyl]-1H-indol-3-yl}etha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -45.0 | n/a | n/a | 148 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21348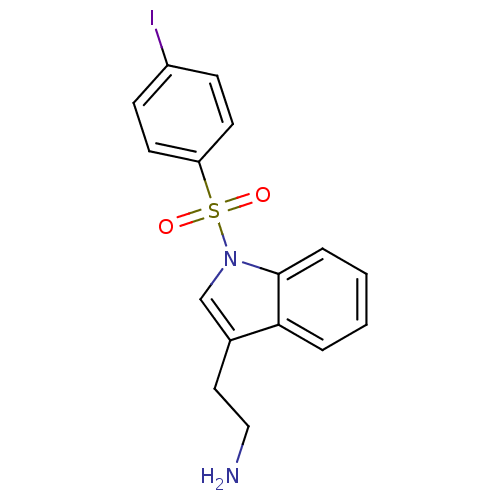 (2-{1-[(4-iodobenzene)sulfonyl]-1H-indol-3-yl}ethan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21344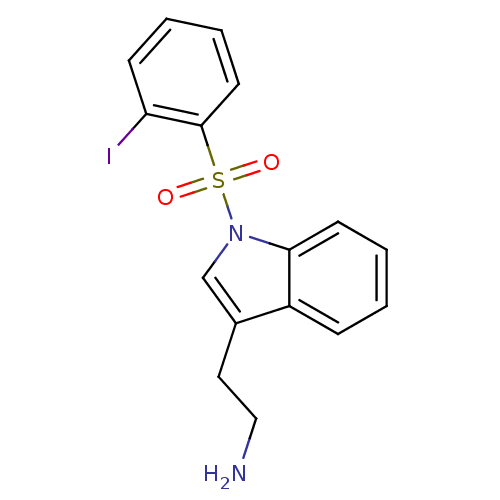 (2-{1-[(2-iodobenzene)sulfonyl]-1H-indol-3-yl}ethan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50129371 (3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]o...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to dopamine transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21346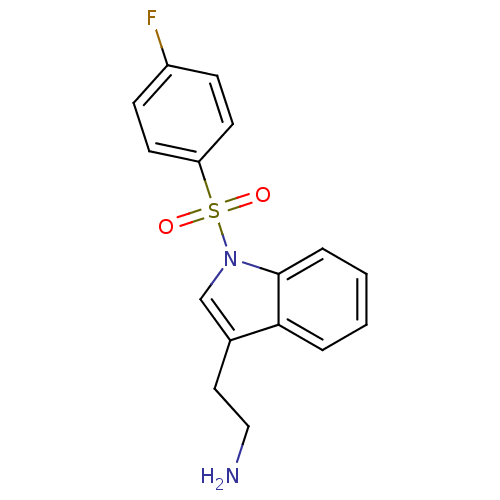 (2-{1-[(4-fluorobenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50129370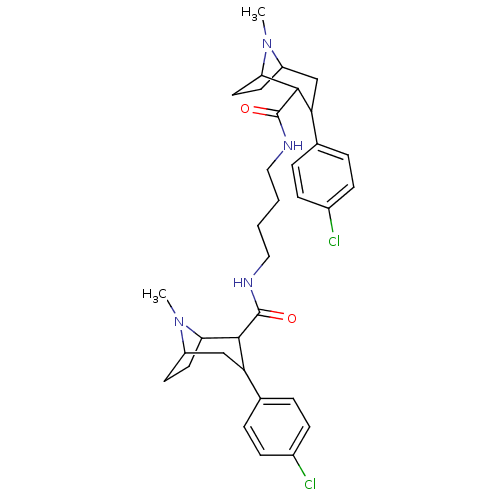 (3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]o...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to dopamine transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21364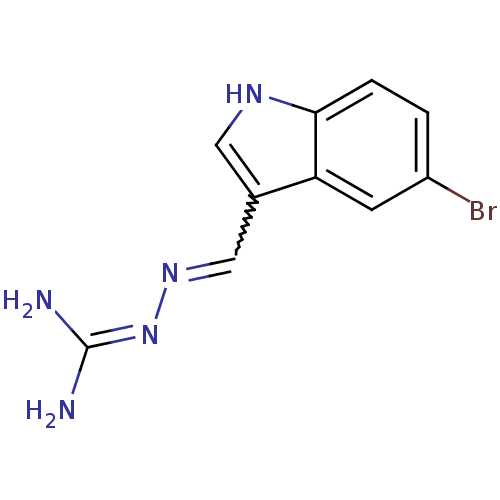 (3-[(E)-[(5-bromo-1H-indol-3-yl)methylidene]amino]g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21352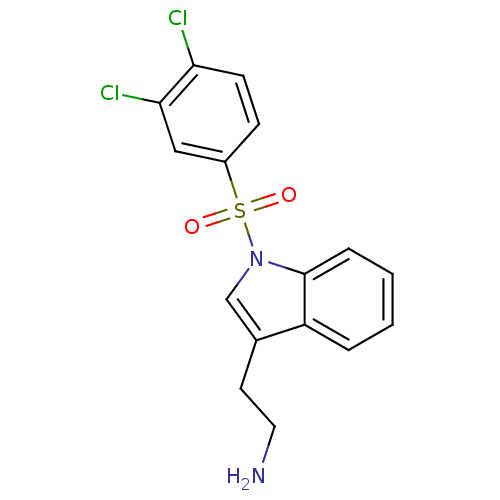 (2-{1-[(3,4-dichlorobenzene)sulfonyl]-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21356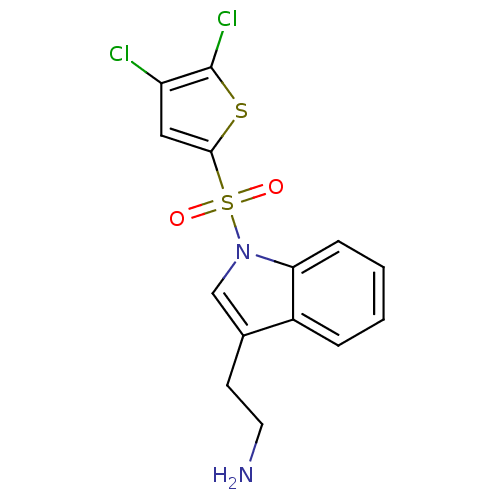 (2-{1-[(4,5-dichlorothiophene-2-)sulfonyl]-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50035738 ((R)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to dopamine transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50162500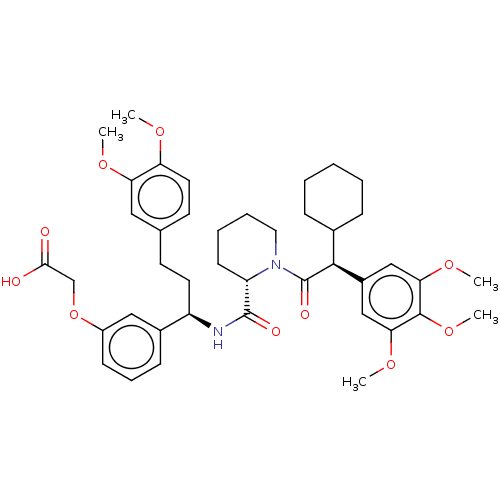 (CHEMBL3792975) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 FK506-binding domain (1 to 140 amino acids) (unknown origin) incubated for 30 mins using fluorescein-conjugated 2-(5-((2-(... | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50035738 ((R)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to Serotonin transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21350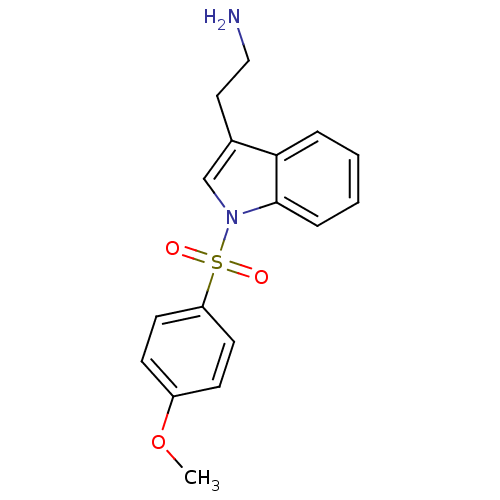 (2-{1-[(4-methoxybenzene)sulfonyl]-1H-indol-3-yl}et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114327 BindingDB Entry DOI: 10.7270/Q2NP28GR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50129368 (3-(4-Chloro-phenyl)--8-methyl-8-aza-bicyclo[3.2.1]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to Serotonin transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50030448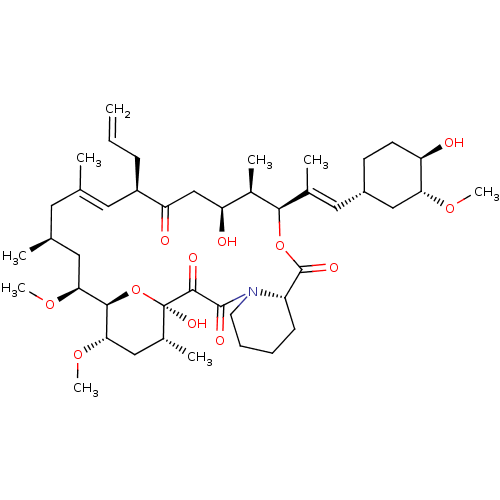 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50125333 (CHEMBL3623612) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP12 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50511138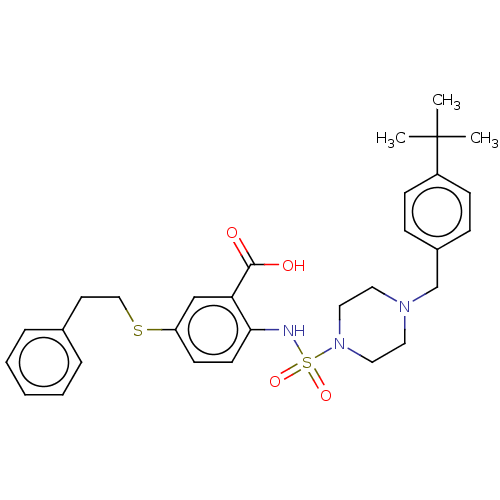 (CHEMBL4576854) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114327 BindingDB Entry DOI: 10.7270/Q2NP28GR | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM81348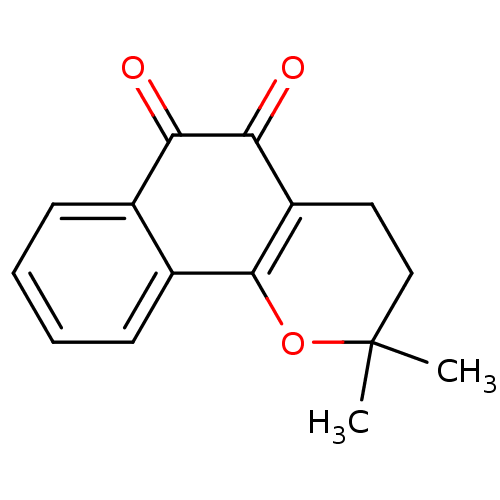 (β-Lapachone (A3) | Beta lapachone | R115 (Rea...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00925 BindingDB Entry DOI: 10.7270/Q28D010N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-related protein A1 (Homo sapiens (Human)) | BDBM50511138 (CHEMBL4576854) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114327 BindingDB Entry DOI: 10.7270/Q2NP28GR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50129371 (3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]o...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to Serotonin transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125332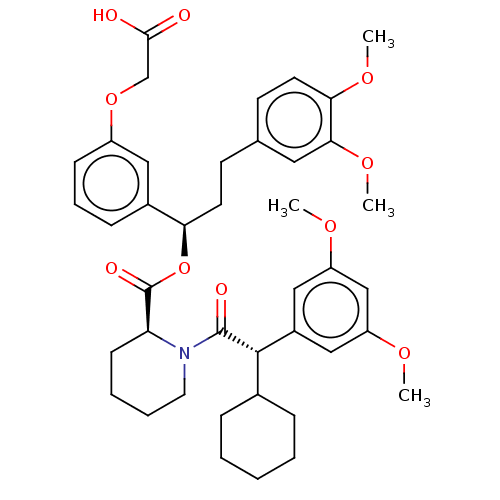 (CHEMBL3623613) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 124 | -39.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50162684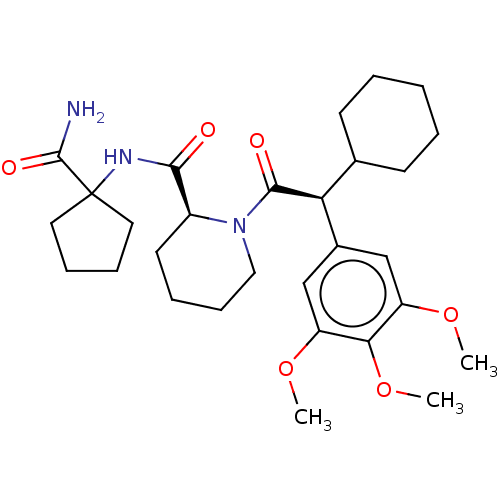 (CHEMBL3793603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 FK506-binding domain (1 to 140 amino acids) (unknown origin) incubated for 30 mins using fluorescein-conjugated 2-(5-((2-(... | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125331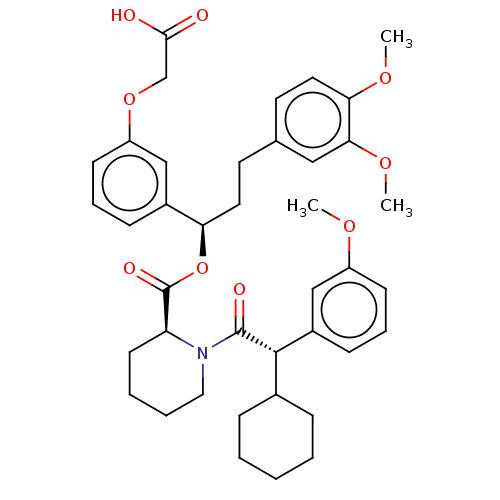 (CHEMBL3623614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50519836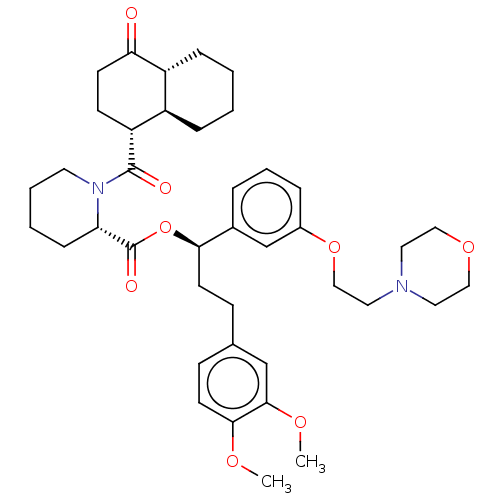 (CHEMBL4588417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged/C-terminal FLAG tagged FKBP51 expressed in Escherichia coli BL21 (DE3) cells incubated for 30 m... | J Med Chem 63: 231-240 (2020) Article DOI: 10.1021/acs.jmedchem.9b01157 BindingDB Entry DOI: 10.7270/Q2Q52T10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bcl-2-like protein 2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114327 BindingDB Entry DOI: 10.7270/Q2NP28GR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22418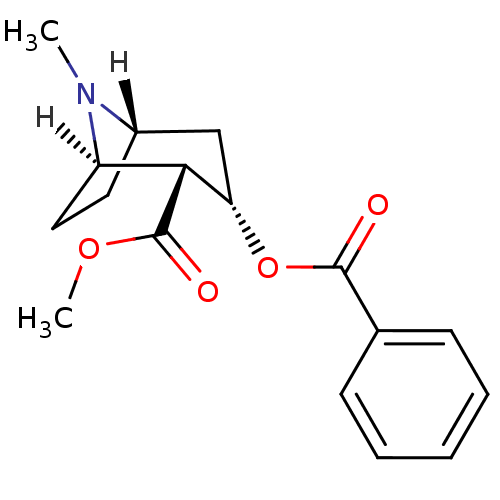 (Cocaine | Cocaine (-) | methyl (1R,2R,3S,5S)-3-(be...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of [125I]RTI-55 binding to Serotonin transporter in HEK cells | Bioorg Med Chem Lett 13: 2151-4 (2003) BindingDB Entry DOI: 10.7270/Q2DN45K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50519830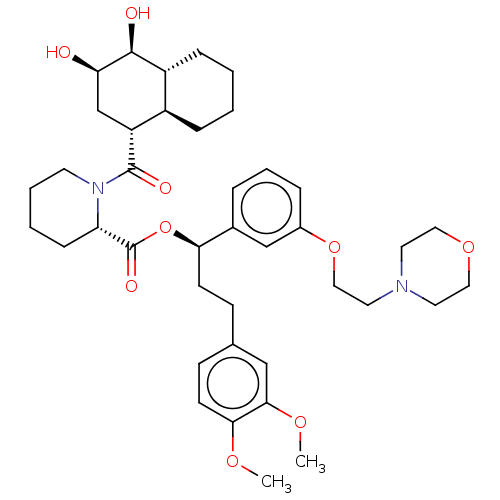 (CHEMBL4463025) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged/C-terminal FLAG tagged FKBP51 expressed in Escherichia coli BL21 (DE3) cells incubated for 30 m... | J Med Chem 63: 231-240 (2020) Article DOI: 10.1021/acs.jmedchem.9b01157 BindingDB Entry DOI: 10.7270/Q2Q52T10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50125326 (CHEMBL3623618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP12 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1135 total ) | Next | Last >> |