| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin G/H synthase 2 |
|---|
| Ligand | BDBM22955 |
|---|
| Substrate/Competitor | BDBM22319 |
|---|
| Meas. Tech. | Time-Dependent Inhibition Assay |
|---|
| Temperature | 310.15±n/a K |
|---|
| IC50 | 50±n/a nM |
|---|
| Comments | extracted |
|---|
| Citation |  Kalgutkar, AS; Crews, BC; Rowlinson, SW; Marnett, AB; Kozak, KR; Remmel, RP; Marnett, LJ Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci U S A97:925-30 (2000) [PubMed] Article Kalgutkar, AS; Crews, BC; Rowlinson, SW; Marnett, AB; Kozak, KR; Remmel, RP; Marnett, LJ Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci U S A97:925-30 (2000) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Prostaglandin G/H synthase 2 |
|---|
| Name: | Prostaglandin G/H synthase 2 |
|---|
| Synonyms: | Cox-2 | Cox2 | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2) | Glucocorticoid-regulated inflammatory cyclooxygenase | Gripghs | Macrophage activation-associated marker protein P71/73 | PES-2 | PGH synthase 2 | PGH2_MOUSE | PGHS-2 | PHS II | Pghs-b | Prostaglandin G/H synthase (cyclooxygenase) | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2 | Ptgs2 | TIS10 protein | Tis10 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 69020.39 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | Q05769 |
|---|
| Residue: | 604 |
|---|
| Sequence: | MLFRAVLLCAALGLSQAANPCCSNPCQNRGECMSTGFDQYKCDCTRTGFYGENCTTPEFL
TRIKLLLKPTPNTVHYILTHFKGVWNIVNNIPFLRSLIMKYVLTSRSYLIDSPPTYNVHY
GYKSWEAFSNLSYYTRALPPVADDCPTPMGVKGNKELPDSKEVLEKVLLRREFIPDPQGS
NMMFAFFAQHFTHQFFKTDHKRGPGFTRGLGHGVDLNHIYGETLDRQHKLRLFKDGKLKY
QVIGGEVYPPTVKDTQVEMIYPPHIPENLQFAVGQEVFGLVPGLMMYATIWLREHNRVCD
ILKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQ
NRIASEFNTLYHWHPLLPDTFNIEDQEYSFKQFLYNNSILLEHGLTQFVESFTRQIAGRV
AGGRNVPIAVQAVAKASIDQSREMKYQSLNEYRKRFSLKPYTSFEELTGEKEMAAELKAL
YSDIDVMELYPALLVEKPRPDAIFGETMVELGAPFSLKGLMGNPICSPQYWKPSTFGGEV
GFKIINTASIQSLICNNVKGCPFTSFNVQDPQPTKTATINASASHSRLDDINPTVLIKRR
STEL
|
|
|
|---|
| BDBM22955 |
|---|
| BDBM22319 |
|---|
| Name | BDBM22955 |
|---|
| Synonyms: | 4-methoxyphenyl 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}acetate | CHEMBL316866 | Indomethacin derivative, 8 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H22ClNO5 |
|---|
| Mol. Mass. | 463.91 |
|---|
| SMILES | COc1ccc(OC(=O)Cc2c(C)n(C(=O)c3ccc(Cl)cc3)c3ccc(OC)cc23)cc1 |
|---|
| Structure | 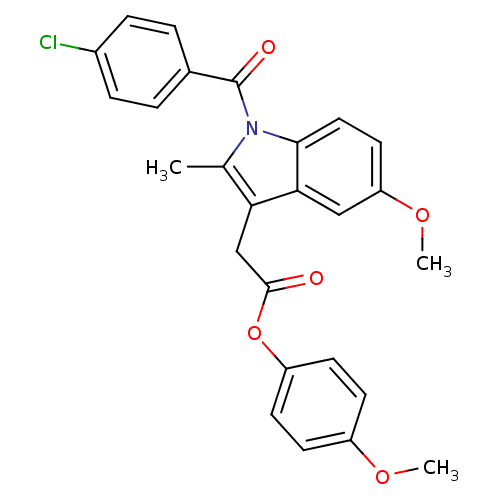
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kalgutkar, AS; Crews, BC; Rowlinson, SW; Marnett, AB; Kozak, KR; Remmel, RP; Marnett, LJ Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci U S A97:925-30 (2000) [PubMed] Article
Kalgutkar, AS; Crews, BC; Rowlinson, SW; Marnett, AB; Kozak, KR; Remmel, RP; Marnett, LJ Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci U S A97:925-30 (2000) [PubMed] Article