| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, inducible |
|---|
| Ligand | BDBM50148162 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | NOS Enzyme Inhibition Assay |
|---|
| pH | 7±0 |
|---|
| Temperature | 310.15±0 K |
|---|
| IC50 | 3.5e+2±n/a nM |
|---|
| Citation |  Garcin, ED; Arvai, AS; Rosenfeld, RJ; Kroeger, MD; Crane, BR; Andersson, G; Andrews, G; Hamley, PJ; Mallinder, PR; Nicholls, DJ; St-Gallay, SA; Tinker, AC; Gensmantel, NP; Mete, A; Cheshire, DR; Connolly, S; Stuehr, DJ; Aberg, A; Wallace, AV; Tainer, JA; Getzoff, ED Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol4:700-7 (2008) [PubMed] Article Garcin, ED; Arvai, AS; Rosenfeld, RJ; Kroeger, MD; Crane, BR; Andersson, G; Andrews, G; Hamley, PJ; Mallinder, PR; Nicholls, DJ; St-Gallay, SA; Tinker, AC; Gensmantel, NP; Mete, A; Cheshire, DR; Connolly, S; Stuehr, DJ; Aberg, A; Wallace, AV; Tainer, JA; Getzoff, ED Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol4:700-7 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Nitric oxide synthase, inducible |
|---|
| Name: | Nitric oxide synthase, inducible |
|---|
| Synonyms: | HEP-NOS | Hepatocyte NOS | Inducible NO synthase | Inducible NOS | NOS type II | NOS2 | NOS2A | NOS2_HUMAN | Nitric oxide synthase, inducible (iNOS) | iNOS |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 131141.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35228 |
|---|
| Residue: | 1153 |
|---|
| Sequence: | MACPWKFLFKTKFHQYAMNGEKDINNNVEKAPCATSSPVTQDDLQYHNLSKQQNESPQPL
VETGKKSPESLVKLDATPLSSPRHVRIKNWGSGMTFQDTLHHKAKGILTCRSKSCLGSIM
TPKSLTRGPRDKPTPPDELLPQAIEFVNQYYGSFKEAKIEEHLARVEAVTKEIETTGTYQ
LTGDELIFATKQAWRNAPRCIGRIQWSNLQVFDARSCSTAREMFEHICRHVRYSTNNGNI
RSAITVFPQRSDGKHDFRVWNAQLIRYAGYQMPDGSIRGDPANVEFTQLCIDLGWKPKYG
RFDVVPLVLQANGRDPELFEIPPDLVLEVAMEHPKYEWFRELELKWYALPAVANMLLEVG
GLEFPGCPFNGWYMGTEIGVRDFCDVQRYNILEEVGRRMGLETHKLASLWKDQAVVEINI
AVLHSFQKQNVTIMDHHSAAESFMKYMQNEYRSRGGCPADWIWLVPPMSGSITPVFHQEM
LNYVLSPFYYYQVEAWKTHVWQDEKRRPKRREIPLKVLVKAVLFACMLMRKTMASRVRVT
ILFATETGKSEALAWDLGALFSCAFNPKVVCMDKYRLSCLEEERLLLVVTSTFGNGDCPG
NGEKLKKSLFMLKELNNKFRYAVFGLGSSMYPRFCAFAHDIDQKLSHLGASQLTPMGEGD
ELSGQEDAFRSWAVQTFKAACETFDVRGKQHIQIPKLYTSNVTWDPHHYRLVQDSQPLDL
SKALSSMHAKNVFTMRLKSRQNLQSPTSSRATILVELSCEDGQGLNYLPGEHLGVCPGNQ
PALVQGILERVVDGPTPHQTVRLEALDESGSYWVSDKRLPPCSLSQALTYFLDITTPPTQ
LLLQKLAQVATEEPERQRLEALCQPSEYSKWKFTNSPTFLEVLEEFPSLRVSAGFLLSQL
PILKPRFYSISSSRDHTPTEIHLTVAVVTYHTRDGQGPLHHGVCSTWLNSLKPQDPVPCF
VRNASGFHLPEDPSHPCILIGPGTGIAPFRSFWQQRLHDSQHKGVRGGRMTLVFGCRRPD
EDHIYQEEMLEMAQKGVLHAVHTAYSRLPGKPKVYVQDILRQQLASEVLRVLHKEPGHLY
VCGDVRMARDVAHTLKQLVAAKLKLNEEQVEDYFFQLKSQKRYHEDIFGAVFPYEAKKDR
VAVQPSSLEMSAL
|
|
|
|---|
| BDBM50148162 |
|---|
| n/a |
|---|
| Name | BDBM50148162 |
|---|
| Synonyms: | 4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carboxylic acid ethyl ester | CHEMBL420671 | ETHYL 4-[(4-METHYLPYRIDIN-2-YL)AMINO]PIPERIDINE-1-CARBOXYLATE | Ethyl 4-[(4-methylpyridin-2-yl)amino]piperidine-1-carboxylate, 9 | ethyl 4-(4-methylpyridin-2-ylamino)piperidine-1-carboxylate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H21N3O2 |
|---|
| Mol. Mass. | 263.3354 |
|---|
| SMILES | CCOC(=O)N1CCC(CC1)Nc1cc(C)ccn1 |
|---|
| Structure | 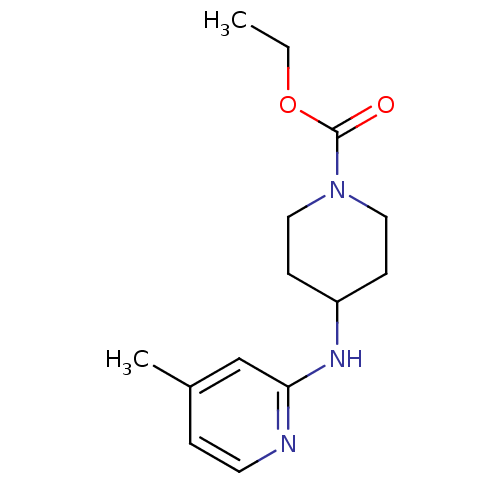
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Garcin, ED; Arvai, AS; Rosenfeld, RJ; Kroeger, MD; Crane, BR; Andersson, G; Andrews, G; Hamley, PJ; Mallinder, PR; Nicholls, DJ; St-Gallay, SA; Tinker, AC; Gensmantel, NP; Mete, A; Cheshire, DR; Connolly, S; Stuehr, DJ; Aberg, A; Wallace, AV; Tainer, JA; Getzoff, ED Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol4:700-7 (2008) [PubMed] Article
Garcin, ED; Arvai, AS; Rosenfeld, RJ; Kroeger, MD; Crane, BR; Andersson, G; Andrews, G; Hamley, PJ; Mallinder, PR; Nicholls, DJ; St-Gallay, SA; Tinker, AC; Gensmantel, NP; Mete, A; Cheshire, DR; Connolly, S; Stuehr, DJ; Aberg, A; Wallace, AV; Tainer, JA; Getzoff, ED Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol4:700-7 (2008) [PubMed] Article