| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50246120 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1676529 (CHEMBL4026672) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Lan, P; Romero, FA; Wodka, D; Kassick, AJ; Dang, Q; Gibson, T; Cashion, D; Zhou, G; Chen, Y; Zhang, X; Zhang, A; Li, Y; Trujillo, ME; Shao, Q; Wu, M; Xu, S; He, H; MacKenna, D; Staunton, J; Chapman, KT; Weber, A; Sebhat, IK; Makara, GM Hit-to-Lead Optimization and Discovery of 5-((5-([1,1'-Biphenyl]-4-yl)-6-chloro-1H-benzo[d]imidazol-2-yl)oxy)-2-methylbenzoic Acid (MK-3903): A Novel Class of Benzimidazole-Based Activators of AMP-Activated Protein Kinase. J Med Chem60:9040-9052 (2017) [PubMed] Article Lan, P; Romero, FA; Wodka, D; Kassick, AJ; Dang, Q; Gibson, T; Cashion, D; Zhou, G; Chen, Y; Zhang, X; Zhang, A; Li, Y; Trujillo, ME; Shao, Q; Wu, M; Xu, S; He, H; MacKenna, D; Staunton, J; Chapman, KT; Weber, A; Sebhat, IK; Makara, GM Hit-to-Lead Optimization and Discovery of 5-((5-([1,1'-Biphenyl]-4-yl)-6-chloro-1H-benzo[d]imidazol-2-yl)oxy)-2-methylbenzoic Acid (MK-3903): A Novel Class of Benzimidazole-Based Activators of AMP-Activated Protein Kinase. J Med Chem60:9040-9052 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50246120 |
|---|
| n/a |
|---|
| Name | BDBM50246120 |
|---|
| Synonyms: | CHEMBL4082745 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H19ClN2O3 |
|---|
| Mol. Mass. | 454.904 |
|---|
| SMILES | Cc1ccc(Oc2nc3cc(c(Cl)cc3[nH]2)-c2ccc(cc2)-c2ccccc2)cc1C(O)=O |
|---|
| Structure | 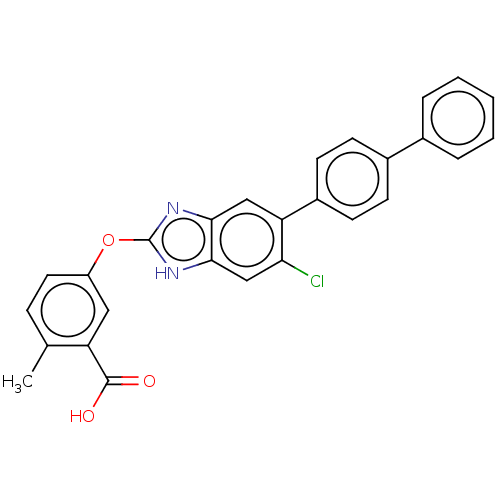
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lan, P; Romero, FA; Wodka, D; Kassick, AJ; Dang, Q; Gibson, T; Cashion, D; Zhou, G; Chen, Y; Zhang, X; Zhang, A; Li, Y; Trujillo, ME; Shao, Q; Wu, M; Xu, S; He, H; MacKenna, D; Staunton, J; Chapman, KT; Weber, A; Sebhat, IK; Makara, GM Hit-to-Lead Optimization and Discovery of 5-((5-([1,1'-Biphenyl]-4-yl)-6-chloro-1H-benzo[d]imidazol-2-yl)oxy)-2-methylbenzoic Acid (MK-3903): A Novel Class of Benzimidazole-Based Activators of AMP-Activated Protein Kinase. J Med Chem60:9040-9052 (2017) [PubMed] Article
Lan, P; Romero, FA; Wodka, D; Kassick, AJ; Dang, Q; Gibson, T; Cashion, D; Zhou, G; Chen, Y; Zhang, X; Zhang, A; Li, Y; Trujillo, ME; Shao, Q; Wu, M; Xu, S; He, H; MacKenna, D; Staunton, J; Chapman, KT; Weber, A; Sebhat, IK; Makara, GM Hit-to-Lead Optimization and Discovery of 5-((5-([1,1'-Biphenyl]-4-yl)-6-chloro-1H-benzo[d]imidazol-2-yl)oxy)-2-methylbenzoic Acid (MK-3903): A Novel Class of Benzimidazole-Based Activators of AMP-Activated Protein Kinase. J Med Chem60:9040-9052 (2017) [PubMed] Article