 Found 11945 hits with Last Name = 'wu' and Initial = 'm'
Found 11945 hits with Last Name = 'wu' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50095611
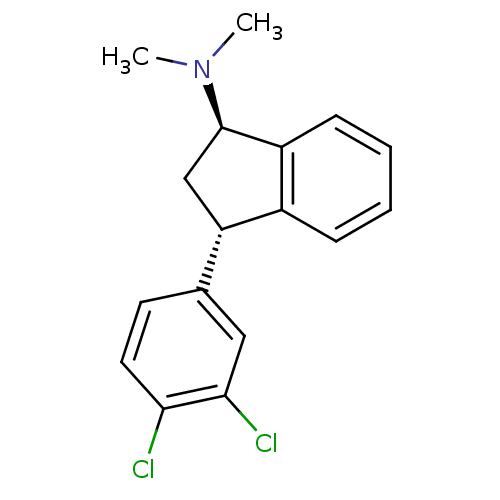
(CHEMBL356750 | [3-(3,4-Dichloro-phenyl)-indan-1-yl...)Show SMILES CN(C)[C@@H]1C[C@H](c2ccccc12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H17Cl2N/c1-20(2)17-10-14(12-5-3-4-6-13(12)17)11-7-8-15(18)16(19)9-11/h3-9,14,17H,10H2,1-2H3/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI |
J Med Chem 43: 4981-92 (2001)
BindingDB Entry DOI: 10.7270/Q2SX6DXD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50095611

(CHEMBL356750 | [3-(3,4-Dichloro-phenyl)-indan-1-yl...)Show SMILES CN(C)[C@@H]1C[C@H](c2ccccc12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H17Cl2N/c1-20(2)17-10-14(12-5-3-4-6-13(12)17)11-7-8-15(18)16(19)9-11/h3-9,14,17H,10H2,1-2H3/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI |
J Med Chem 43: 4981-92 (2001)
BindingDB Entry DOI: 10.7270/Q2SX6DXD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
Bioorg Med Chem Lett 17: 1523-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.007
BindingDB Entry DOI: 10.7270/Q22J6950 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50579244

(Avoralstat | BCX-4161)Show SMILES COc1cc(c(cc1C=C)C(=O)Nc1ccc(cc1)C(N)=N)-c1ccc(nc1C(O)=O)C(=O)NCC1CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00511
BindingDB Entry DOI: 10.7270/Q29C7288 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50369770
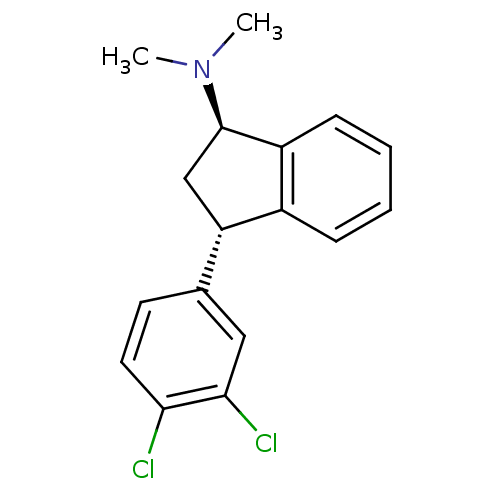
(CHEMBL1788140)Show SMILES CN(C)[C@@H]1C[C@H](c2ccccc12)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20(2)17-10-14(12-5-3-4-6-13(12)17)11-7-8-15(18)16(19)9-11/h3-9,14,17H,10H2,1-2H3/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI |
J Med Chem 43: 4981-92 (2001)
BindingDB Entry DOI: 10.7270/Q2SX6DXD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588206

(CHEMBL5183968)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c-2c(-[#8]C([#6])([#6])c3ccc(-[#6]-[#8])cc-23)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588208
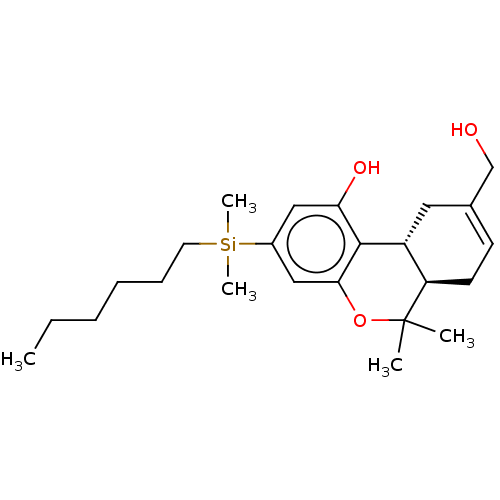
(CHEMBL5187099)Show SMILES [H][C@@]12[#6]-[#6](-[#6]-[#8])=[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6]-[#6]-[#6] |r,c:5| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM416926

((+)-1-(3-(aminomethyl)phenyl)-N-(5-((3-cyanophenyl...)Show SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1cc(ccc1F)C(NCC1CC1)c1cccc(c1)C#N)C(F)(F)F Show InChI InChI=1S/C30H26F4N6O/c31-24-10-9-22(28(37-17-18-7-8-18)21-5-1-3-19(11-21)15-35)13-25(24)38-29(41)26-14-27(30(32,33)34)39-40(26)23-6-2-4-20(12-23)16-36/h1-6,9-14,18,28,37H,7-8,16-17,36H2,(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00511
BindingDB Entry DOI: 10.7270/Q29C7288 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389603

(CHEMBL2069499)Show SMILES CCCCC1(CCCC)N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SST3 receptor expressed in CHO cells after 60 mins by scintillation counting |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50588209
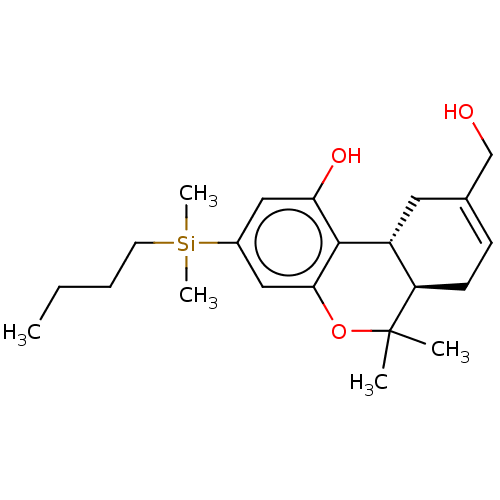
(CHEMBL5178491)Show SMILES [H][C@@]12[#6]-[#6](-[#6]-[#8])=[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6] |r,c:5| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588196
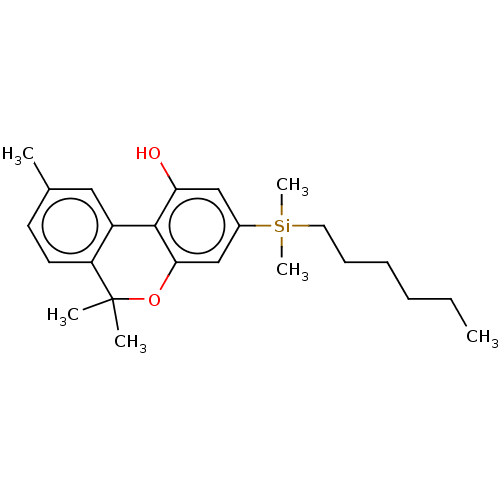
(CHEMBL5194443)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c-2c(-[#8]C([#6])([#6])c3ccc(-[#6])cc-23)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588198
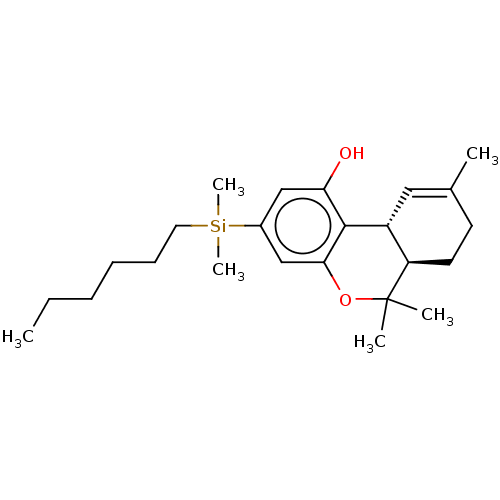
(CHEMBL5200844)Show SMILES [H][C@@]12[#6]=[#6](-[#6])-[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6]-[#6]-[#6] |r,t:2| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human full length carbonic anhydrase 7 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human full length carbonic anhydrase 6 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18216
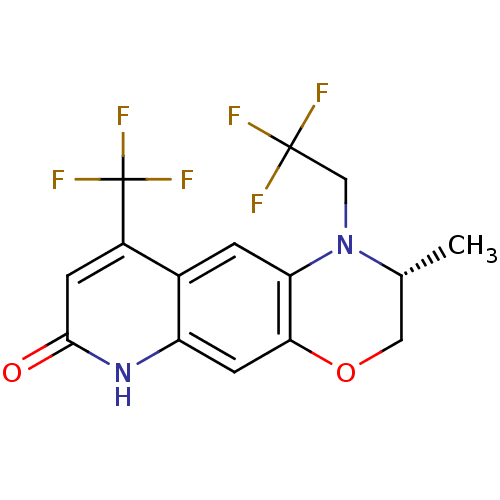
((2R)-2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluor...)Show SMILES C[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C15H12F6N2O2/c1-7-5-25-12-4-10-8(2-11(12)23(7)6-14(16,17)18)9(15(19,20)21)3-13(24)22-10/h2-4,7H,5-6H2,1H3,(H,22,24)/t7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50588208

(CHEMBL5187099)Show SMILES [H][C@@]12[#6]-[#6](-[#6]-[#8])=[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6]-[#6]-[#6] |r,c:5| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50280339

((S)-2-[4-(2-Benzenesulfonyl-ethoxy)-3-methoxy-5-(p...)Show SMILES CCCS(=O)(=O)c1cc(cc(OC)c1OCCS(=O)(=O)c1ccccc1)C1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C31H38O10S2/c1-6-15-43(34,35)29-20-22(19-28(38-4)31(29)40-14-16-42(32,33)23-10-8-7-9-11-23)25-13-12-24(41-25)21-17-26(36-2)30(39-5)27(18-21)37-3/h7-11,17-20,24-25H,6,12-16H2,1-5H3/t24-,25?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of binding of [3H]-C18 PAF to human platelet membrane Platelet activating factor receptor |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha [200-419]/gamma [183-417]
(Homo sapiens (Human)) | BDBM36810
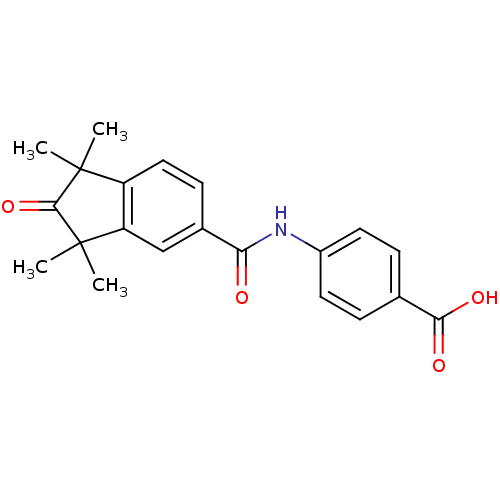
(BMS753 | US9963439, BMS753)Show SMILES CC1(C)C(=O)C(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H21NO4/c1-20(2)15-10-7-13(11-16(15)21(3,4)19(20)26)17(23)22-14-8-5-12(6-9-14)18(24)25/h5-11H,1-4H3,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 4 |
Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC)
| Assay Description
Competitive assay were perform with 5nM tritiated all-trans retinoic acid (t-RA; 5nM) with or without 100-fold excess of non-radioactive t-RA (500nM)... |
Chem Biol 6: 519-29 (1999)
Article DOI: 10.1016/S1074-5521(99)80084-2
BindingDB Entry DOI: 10.7270/Q2CV4G39 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha [200-419]/gamma [183-417]
(Homo sapiens (Human)) | BDBM36811

(BMS614)Show SMILES CC1(C)CC=C(c2cnc3ccccc3c2)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O |t:4| Show InChI InChI=1S/C29H24N2O3/c1-29(2)14-13-23(21-15-19-5-3-4-6-26(19)30-17-21)24-16-20(9-12-25(24)29)27(32)31-22-10-7-18(8-11-22)28(33)34/h3-13,15-17H,14H2,1-2H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 4 |
Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC)
| Assay Description
Competitive assay were perform with 5nM tritiated all-trans retinoic acid (t-RA; 5nM) with or without 100-fold excess of non-radioactive t-RA (500nM)... |
Chem Biol 6: 519-29 (1999)
Article DOI: 10.1016/S1074-5521(99)80084-2
BindingDB Entry DOI: 10.7270/Q2CV4G39 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377413

(CHEMBL257379)Show SMILES Cc1cccc(c1)[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H16F6N2O2/c1-11-3-2-4-12(5-11)17-9-31-18-8-15-13(6-16(18)29(17)10-20(22,23)24)14(21(25,26)27)7-19(30)28-15/h2-8,17H,9-10H2,1H3,(H,28,30)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377414
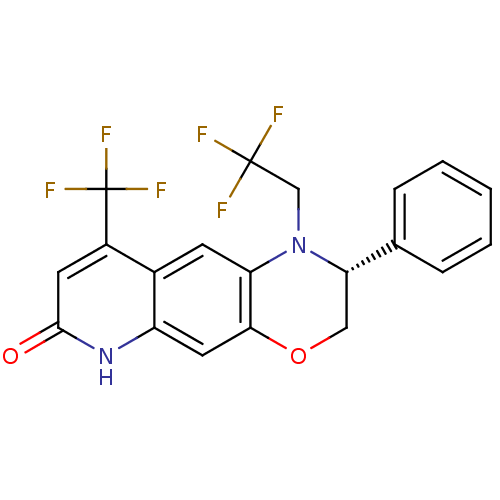
(CHEMBL402835)Show SMILES FC(F)(F)CN1[C@@H](COc2cc3[nH]c(=O)cc(c3cc12)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C20H14F6N2O2/c21-19(22,23)10-28-15-6-12-13(20(24,25)26)7-18(29)27-14(12)8-17(15)30-9-16(28)11-4-2-1-3-5-11/h1-8,16H,9-10H2,(H,27,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50588206

(CHEMBL5183968)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c-2c(-[#8]C([#6])([#6])c3ccc(-[#6]-[#8])cc-23)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma [183-417]
(Homo sapiens (Human)) | BDBM31889
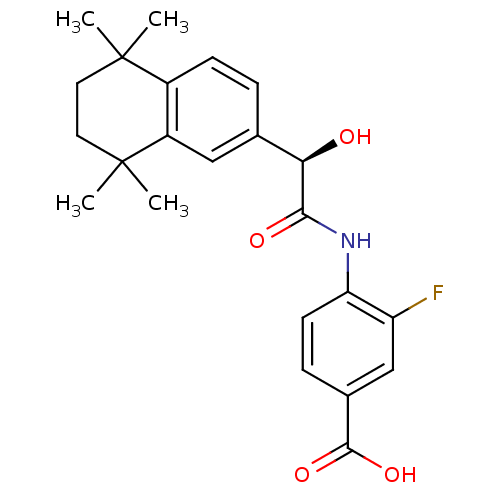
(BMS 961 | BMS270394 | BMS961)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)[C@@H](O)C(=O)Nc1ccc(cc1F)C(O)=O Show InChI InChI=1S/C23H26FNO4/c1-22(2)9-10-23(3,4)16-11-13(5-7-15(16)22)19(26)20(27)25-18-8-6-14(21(28)29)12-17(18)24/h5-8,11-12,19,26H,9-10H2,1-4H3,(H,25,27)(H,28,29)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 1.5 | -46.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 4 |
Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC)
| Assay Description
Competitive assay were perform with 5nM tritiated all-trans retinoic acid (t-RA; 5nM) with or without 100-fold excess of non-radioactive t-RA (500nM)... |
Chem Biol 6: 519-29 (1999)
Article DOI: 10.1016/S1074-5521(99)80084-2
BindingDB Entry DOI: 10.7270/Q2CV4G39 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50588199
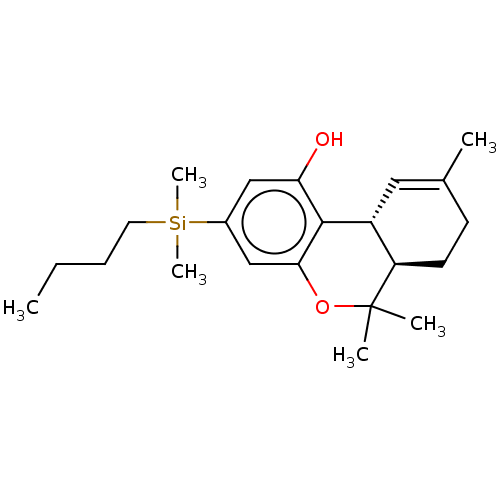
(CHEMBL5189892)Show SMILES [H][C@@]12[#6]=[#6](-[#6])-[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6] |r,t:2| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588201
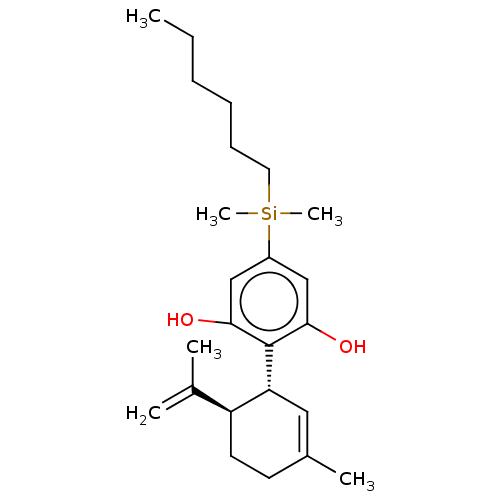
(CHEMBL5204553)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c(-[#6@@H]-2-[#6]=[#6](-[#6])-[#6]-[#6]-[#6@H]-2-[#6](-[#6])=[#6])c(-[#8])c1 |r,t:15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389592
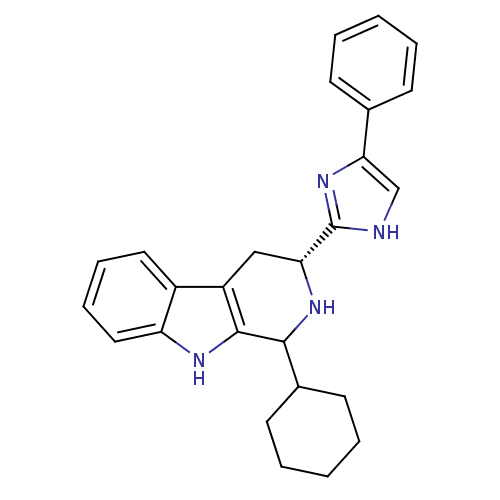
(CHEMBL2069500)Show SMILES C1CCC(CC1)C1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N4/c1-3-9-17(10-4-1)23-16-27-26(30-23)22-15-20-19-13-7-8-14-21(19)28-25(20)24(29-22)18-11-5-2-6-12-18/h1,3-4,7-10,13-14,16,18,22,24,28-29H,2,5-6,11-12,15H2,(H,27,30)/t22-,24?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human SST3 receptor |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha [200-419]
(Homo sapiens (Human)) | BDBM36810

(BMS753 | US9963439, BMS753)Show SMILES CC1(C)C(=O)C(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H21NO4/c1-20(2)15-10-7-13(11-16(15)21(3,4)19(20)26)17(23)22-14-8-5-12(6-9-14)18(24)25/h5-11H,1-4H3,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 4 |
Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC)
| Assay Description
Competitive assay were perform with 5nM tritiated all-trans retinoic acid (t-RA; 5nM) with or without 100-fold excess of non-radioactive t-RA (500nM)... |
Chem Biol 6: 519-29 (1999)
Article DOI: 10.1016/S1074-5521(99)80084-2
BindingDB Entry DOI: 10.7270/Q2CV4G39 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50095629
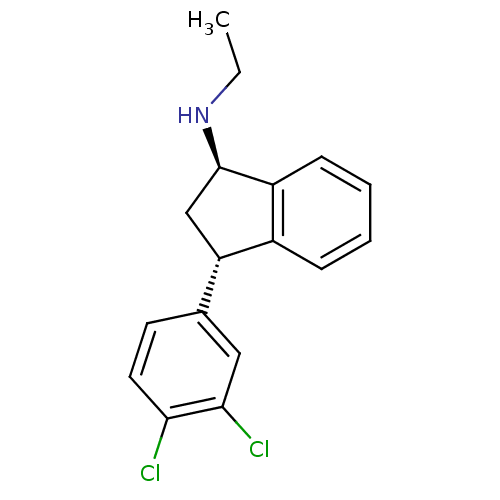
(CHEMBL147983 | [3-(3,4-Dichloro-phenyl)-indan-1-yl...)Show SMILES CCN[C@@H]1C[C@H](c2ccccc12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H17Cl2N/c1-2-20-17-10-14(12-5-3-4-6-13(12)17)11-7-8-15(18)16(19)9-11/h3-9,14,17,20H,2,10H2,1H3/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI |
J Med Chem 43: 4981-92 (2001)
BindingDB Entry DOI: 10.7270/Q2SX6DXD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human full length carbonic anhydrase 7 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50588196

(CHEMBL5194443)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c-2c(-[#8]C([#6])([#6])c3ccc(-[#6])cc-23)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588209

(CHEMBL5178491)Show SMILES [H][C@@]12[#6]-[#6](-[#6]-[#8])=[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6] |r,c:5| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588199

(CHEMBL5189892)Show SMILES [H][C@@]12[#6]=[#6](-[#6])-[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6] |r,t:2| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377415
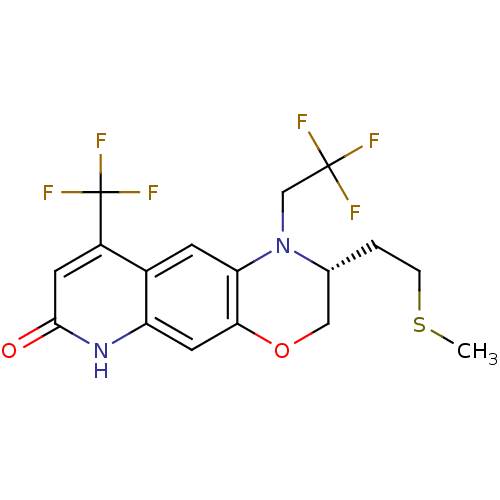
(CHEMBL404483)Show SMILES CSCC[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H16F6N2O2S/c1-28-3-2-9-7-27-14-6-12-10(4-13(14)25(9)8-16(18,19)20)11(17(21,22)23)5-15(26)24-12/h4-6,9H,2-3,7-8H2,1H3,(H,24,26)/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50095619
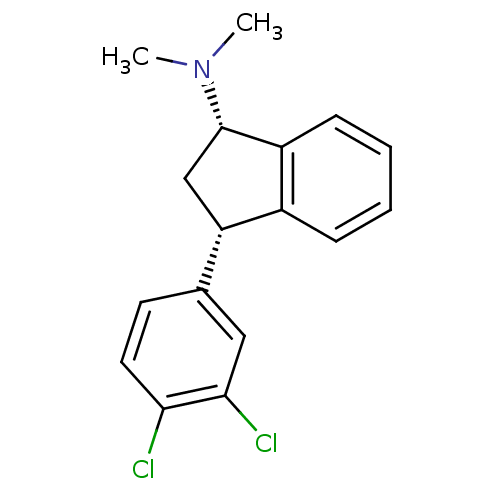
(CHEMBL147950 | [3-(3,4-Dichloro-phenyl)-indan-1-yl...)Show SMILES CN(C)[C@H]1C[C@H](c2ccccc12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H17Cl2N/c1-20(2)17-10-14(12-5-3-4-6-13(12)17)11-7-8-15(18)16(19)9-11/h3-9,14,17H,10H2,1-2H3/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI |
J Med Chem 43: 4981-92 (2001)
BindingDB Entry DOI: 10.7270/Q2SX6DXD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human full length carbonic anhydrase 7 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha [200-419]
(Homo sapiens (Human)) | BDBM36811

(BMS614)Show SMILES CC1(C)CC=C(c2cnc3ccccc3c2)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O |t:4| Show InChI InChI=1S/C29H24N2O3/c1-29(2)14-13-23(21-15-19-5-3-4-6-26(19)30-17-21)24-16-20(9-12-25(24)29)27(32)31-22-10-7-18(8-11-22)28(33)34/h3-13,15-17H,14H2,1-2H3,(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| MMDB
Article
PubMed
| 2.5 | -45.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 4 |
Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC)
| Assay Description
Competitive assay were perform with 5nM tritiated all-trans retinoic acid (t-RA; 5nM) with or without 100-fold excess of non-radioactive t-RA (500nM)... |
Chem Biol 6: 519-29 (1999)
Article DOI: 10.1016/S1074-5521(99)80084-2
BindingDB Entry DOI: 10.7270/Q2CV4G39 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377419
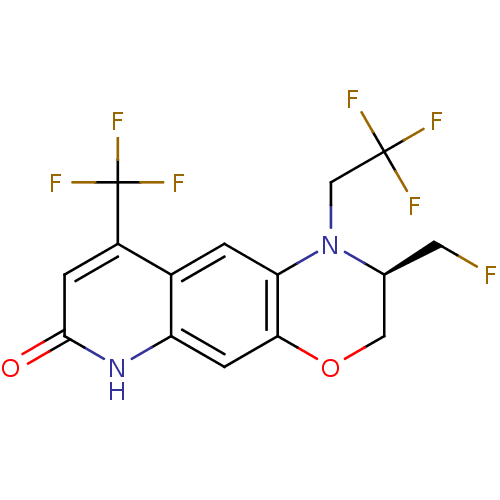
(CHEMBL429311)Show SMILES FC[C@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H11F7N2O2/c16-4-7-5-26-12-3-10-8(1-11(12)24(7)6-14(17,18)19)9(15(20,21)22)2-13(25)23-10/h1-3,7H,4-6H2,(H,23,25)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588207
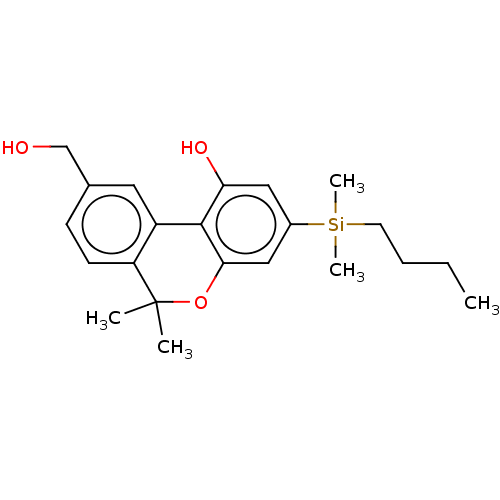
(CHEMBL5192756)Show SMILES [#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c-2c(-[#8]C([#6])([#6])c3ccc(-[#6]-[#8])cc-23)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human full length carbonic anhydrase 7 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human catalytic domain carbonic anhydrase 12 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50297764

((R)-5-cyano-3-(3,4-dioxo-2-(1-phenylpropylamino)cy...)Show SMILES CC[C@@H](Nc1c(Nc2cc(cc(C(=O)N(C)C)c2O)C#N)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H22N4O4/c1-4-16(14-8-6-5-7-9-14)25-18-19(22(30)21(18)29)26-17-11-13(12-24)10-15(20(17)28)23(31)27(2)3/h5-11,16,25-26,28H,4H2,1-3H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human full length carbonic anhydrase 2 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human catalytic domain carbonic anhydrase 12 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50588207

(CHEMBL5192756)Show SMILES [#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c-2c(-[#8]C([#6])([#6])c3ccc(-[#6]-[#8])cc-23)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human full length carbonic anhydrase 7 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human catalytic domain carbonic anhydrase 12 by stopped flow CO2 hydrase assay |
Bioorg Med Chem Lett 18: 2669-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.023
BindingDB Entry DOI: 10.7270/Q23R0TRX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50297761
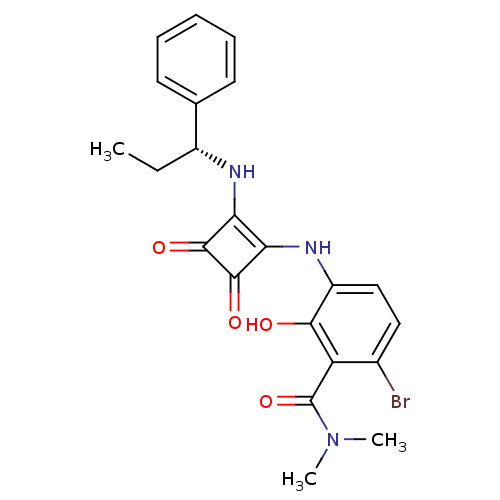
((R)-6-bromo-3-(3,4-dioxo-2-(1-phenylpropylamino)cy...)Show SMILES CC[C@@H](Nc1c(Nc2ccc(Br)c(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C22H22BrN3O4/c1-4-14(12-8-6-5-7-9-12)24-17-18(21(29)20(17)28)25-15-11-10-13(23)16(19(15)27)22(30)26(2)3/h5-11,14,24-25,27H,4H2,1-3H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data