

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402775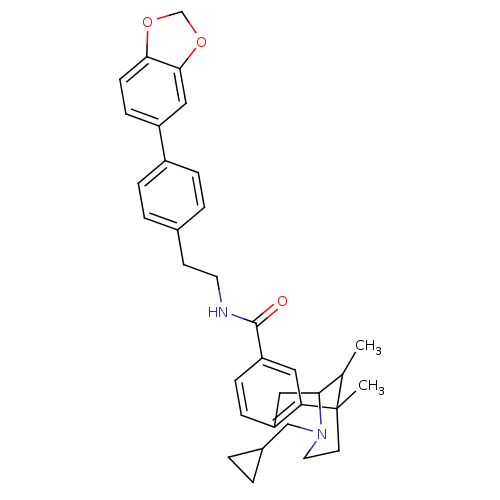 (CHEMBL2208351) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402777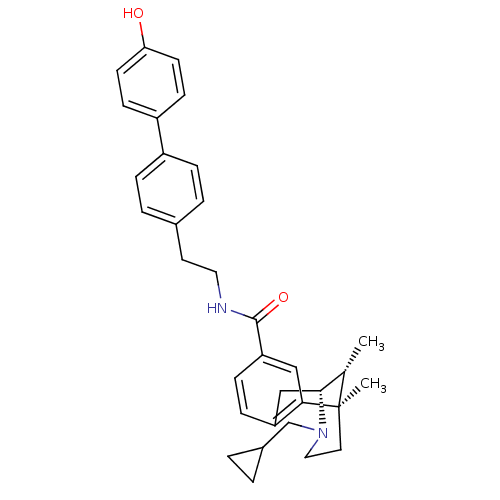 (CHEMBL2208347) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9182 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9180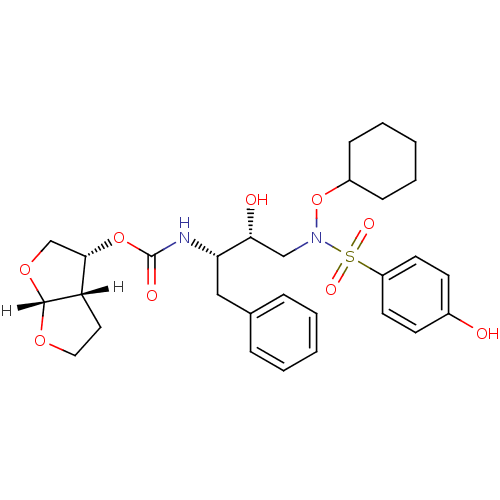 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9171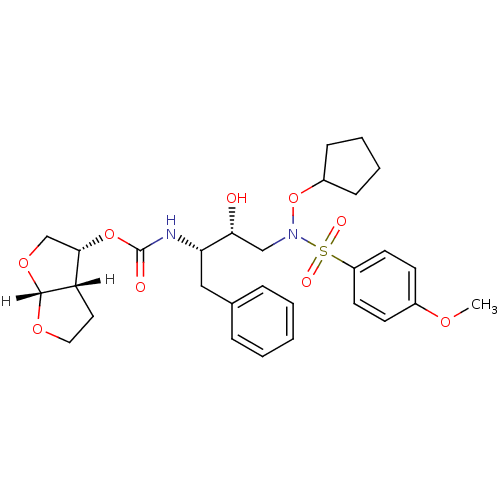 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | <-64.5 | 6 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9175 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9176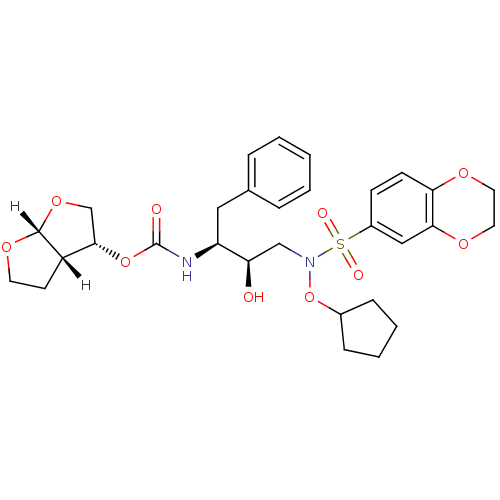 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9173 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9178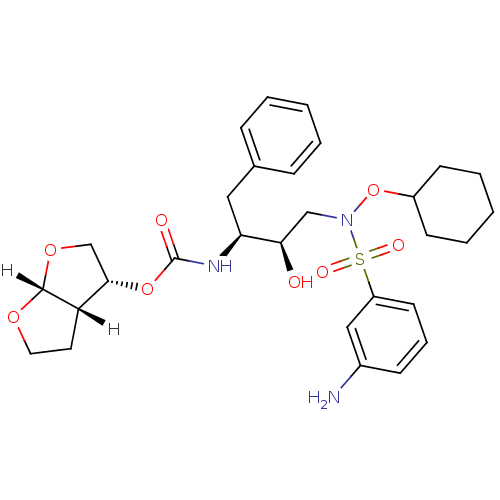 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9174 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9181 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9172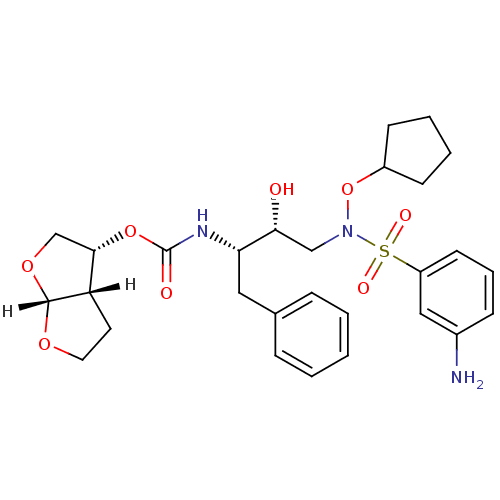 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.00500 | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9177 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.00500 | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9170 ((2S)-N-[(2S,3R)-4-[(cyclopentyloxy)(4-methoxybenze...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.00500 | <-64.5 | 24 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402777 (CHEMBL2208347) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402776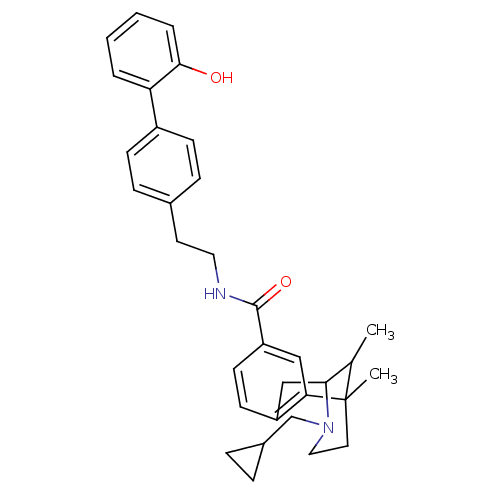 (CHEMBL2208349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402772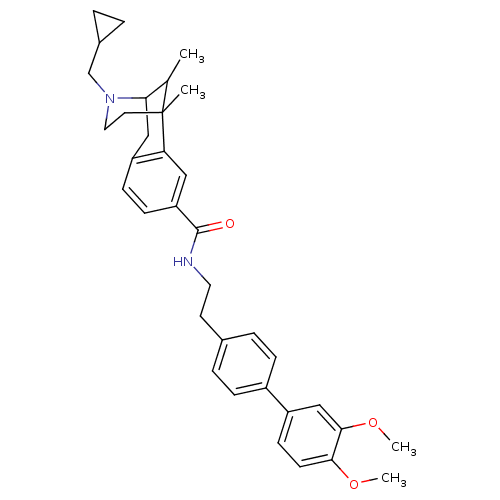 (CHEMBL2208350) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573157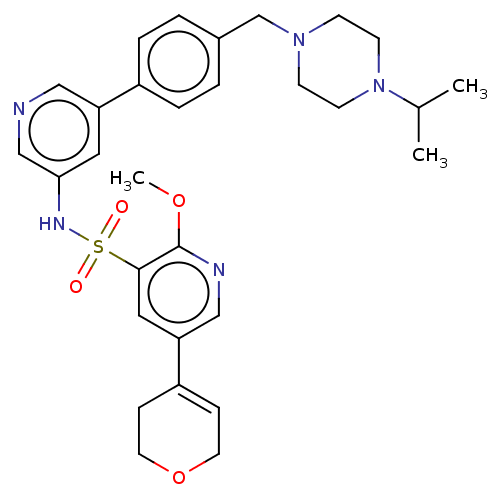 (CHEMBL4850297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573166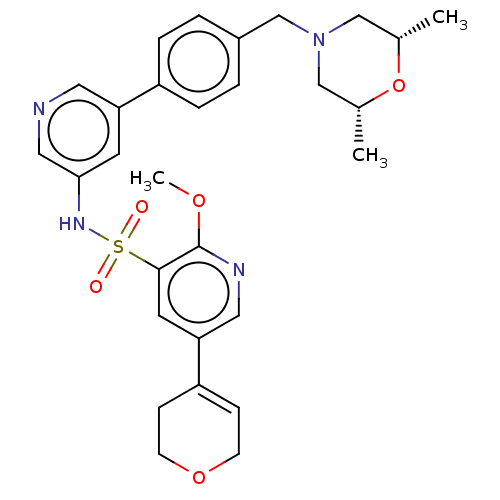 (CHEMBL4869783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573177 (CHEMBL4167702) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50254566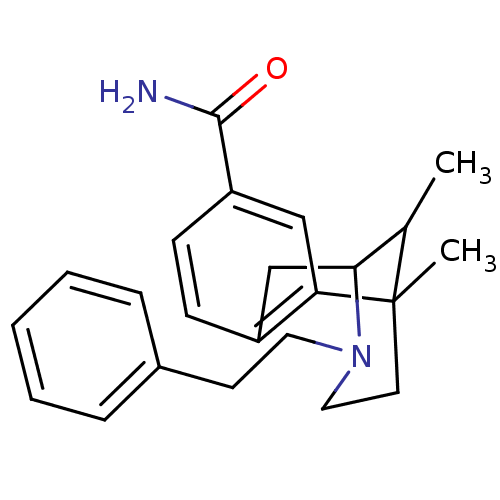 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO form human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573167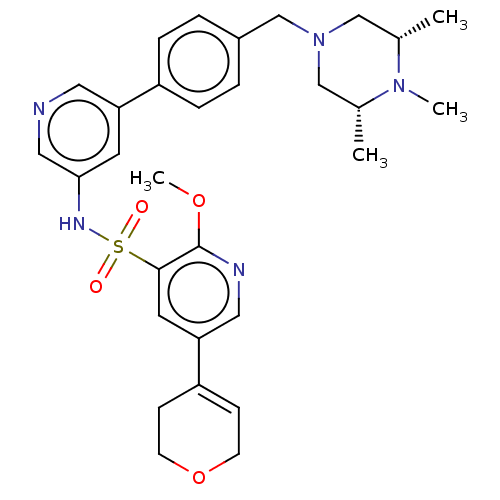 (CHEMBL4858875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9179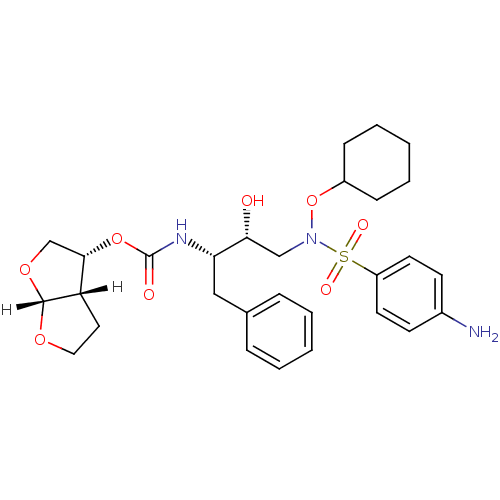 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402773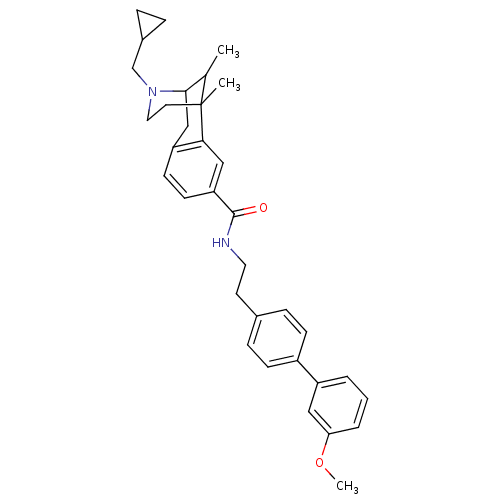 (CHEMBL2208358) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402780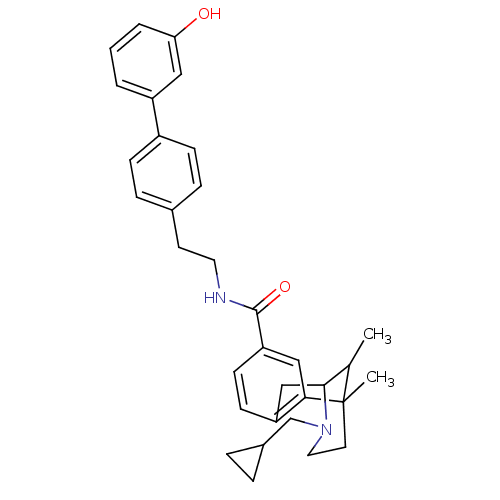 (CHEMBL2208348) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50278436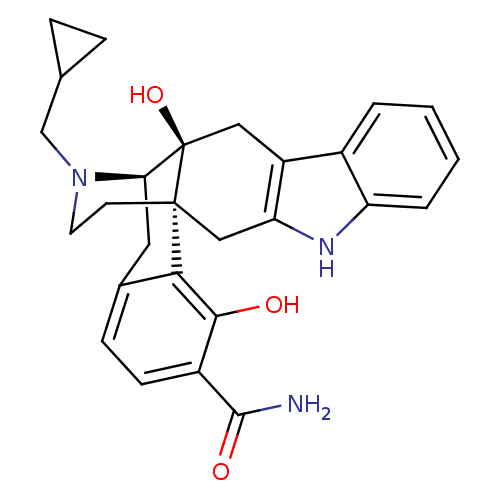 ((1R,13S,14R)-24-(cyclopropylmethyl)-13,20-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573164 (CHEMBL4873390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573182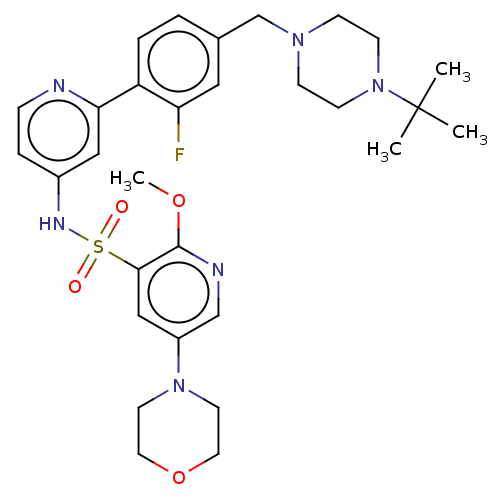 (CHEMBL4175571) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573181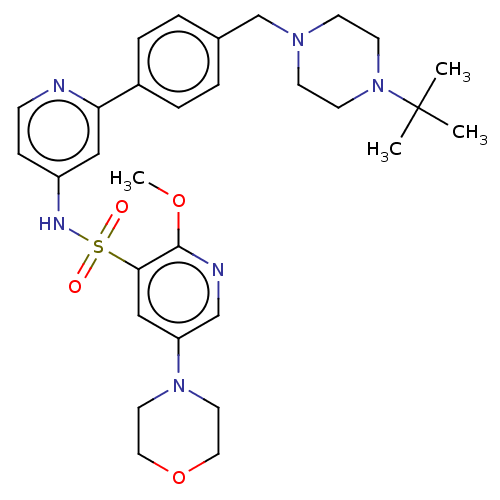 (CHEMBL4165185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551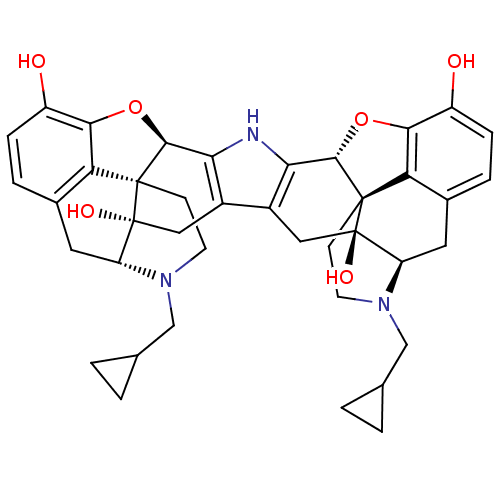 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Activity at human cloned kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573180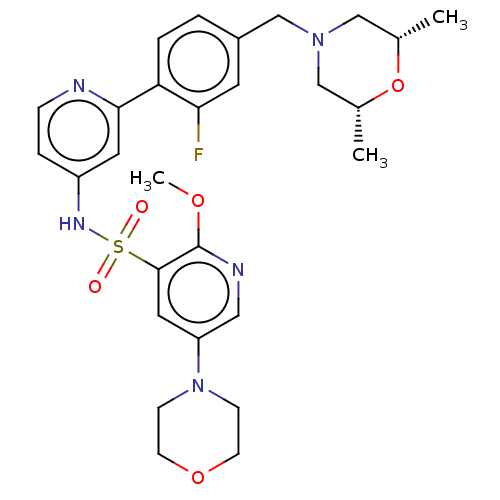 (CHEMBL4175737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 using [3H]-U-69,593 as radioligand in guinea pig brain membranes. | J Med Chem 47: 165-74 (2003) Article DOI: 10.1021/jm0304156 BindingDB Entry DOI: 10.7270/Q2KD1XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573175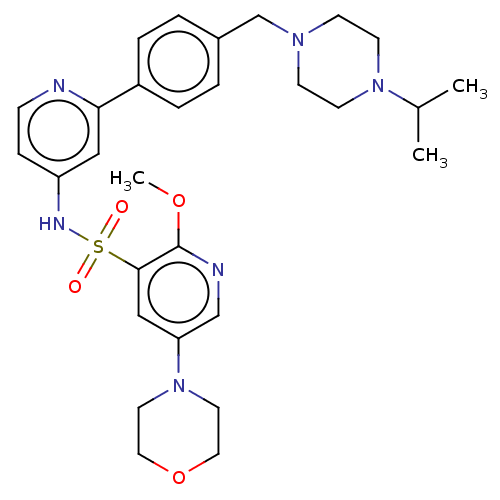 (CHEMBL4169192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0400 | -59.3 | 150 | n/a | n/a | n/a | n/a | 6.8 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 15: 3560-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.101 BindingDB Entry DOI: 10.7270/Q2F769SC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50063687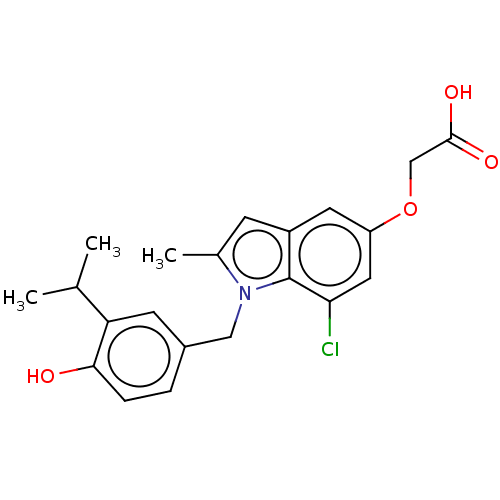 (CHEMBL3397339) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... | Bioorg Med Chem Lett 25: 1377-80 (2015) Article DOI: 10.1016/j.bmcl.2015.02.062 BindingDB Entry DOI: 10.7270/Q2PV6N2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50266003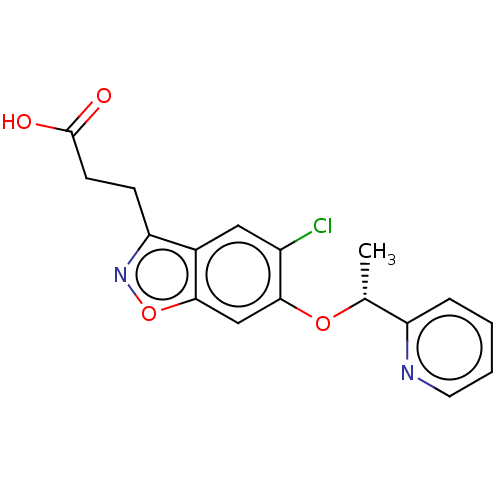 (CHEMBL4091152) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate ... | J Med Chem 60: 3383-3404 (2017) Article DOI: 10.1021/acs.jmedchem.7b00055 BindingDB Entry DOI: 10.7270/Q26112SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277113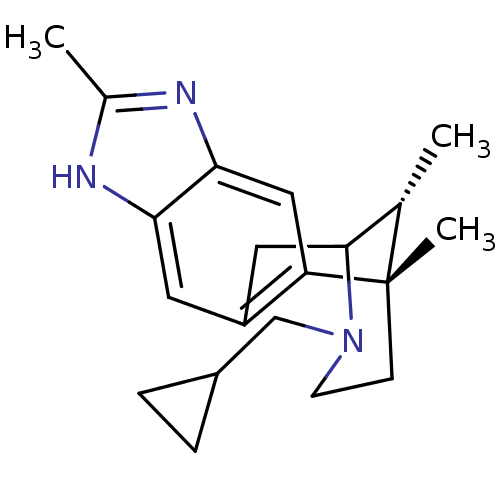 ((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419334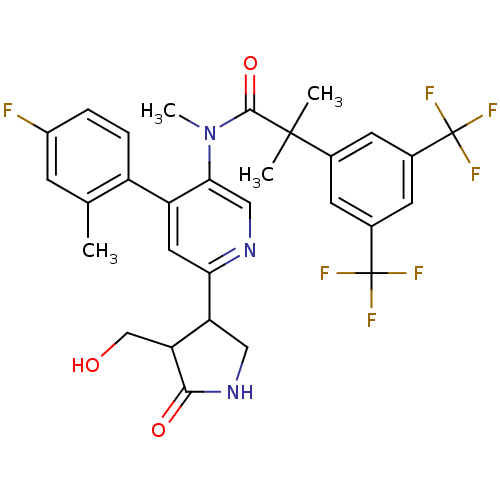 (CHEMBL1911965 | CHEMBL1911967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573179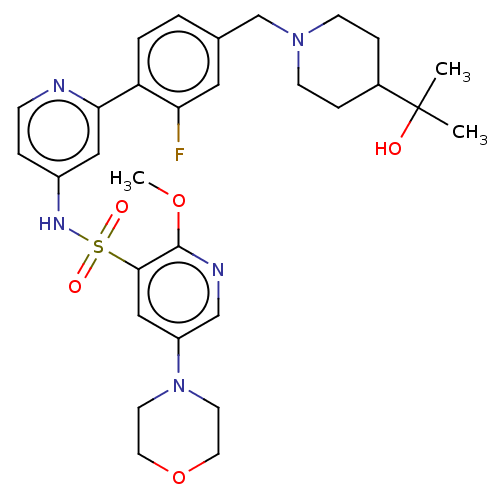 (CHEMBL4174874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50573176 (CHEMBL4173087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01102 BindingDB Entry DOI: 10.7270/Q2N87FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50254568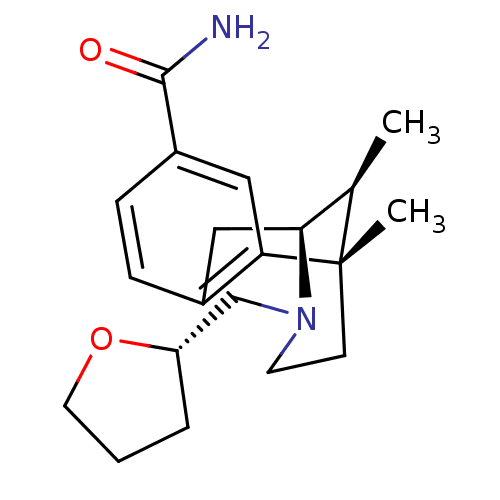 ((2S,6S,11S)-6,11-Dimethyl-3-[(S)-1-(tetrahydro-fur...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO form mu opioid receptor in guinea pig brain | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50165049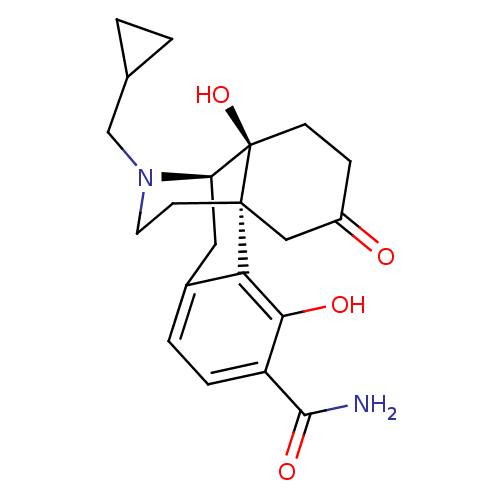 (17-cyclopropylmethyl-3,10-dihydroxy-13-oxo-(1R,9R,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 expressed in CHO cells using [3H]DAMGO as radioligand | Bioorg Med Chem Lett 15: 2107-10 (2005) Article DOI: 10.1016/j.bmcl.2005.02.032 BindingDB Entry DOI: 10.7270/Q2C24X63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278265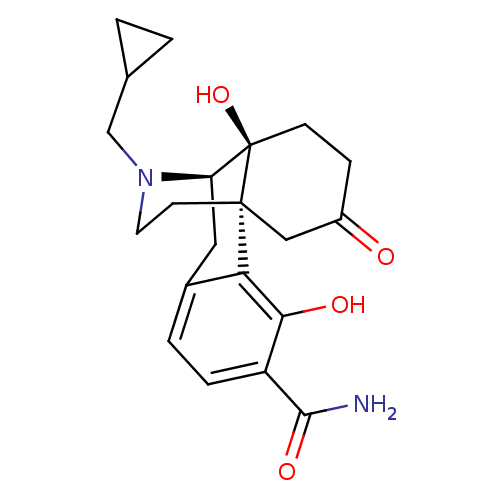 (CCDC 710249, HCl salt | CHEMBL471243) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023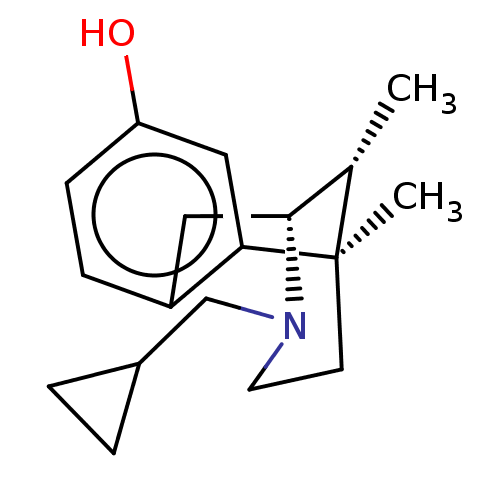 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity to displace radioligand [3H]U-69593 on Opioid receptor kappa 1 in guinea pig membranes. | J Med Chem 43: 3558-65 (2000) BindingDB Entry DOI: 10.7270/Q269749X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity was determined against Opioid receptor kappa 1 obtained from guinea pig brain membranes using [3H]U-69593 as radioligand | Bioorg Med Chem Lett 10: 183-7 (2000) BindingDB Entry DOI: 10.7270/Q2571CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain membranes | Bioorg Med Chem Lett 11: 623-6 (2001) BindingDB Entry DOI: 10.7270/Q2542MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402774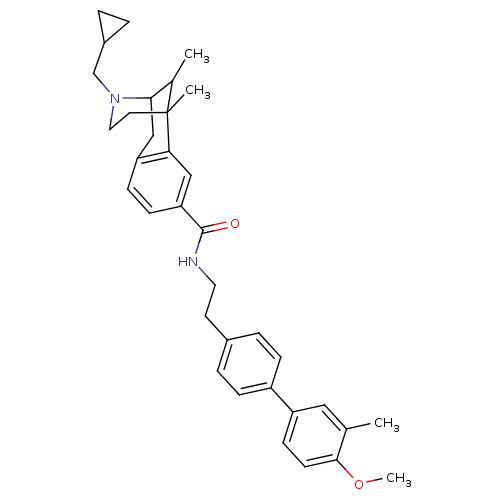 (CHEMBL2208352) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090002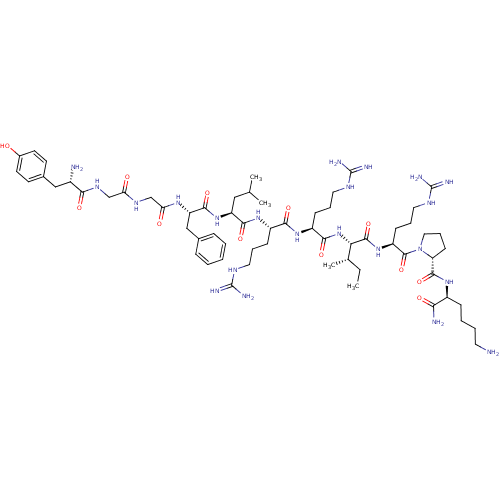 (CHEMBL411003 | Dynorphin A (1-11)-NH2H-Tyr-Gly-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description In vitro binding affinity towards human Opioid receptor kappa 1 on CHO cell membranes using [3H]diprenorphine displacement. | J Med Chem 46: 2104-9 (2003) Article DOI: 10.1021/jm020125+ BindingDB Entry DOI: 10.7270/Q2NZ88CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290071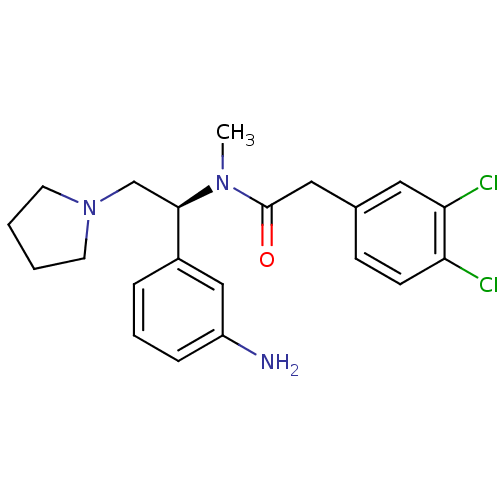 (CHEMBL542149 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 25239 total ) | Next | Last >> |