| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nicotinamide phosphoribosyltransferase |
|---|
| Ligand | BDBM50254186 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_1686176 |
|---|
| Ki | 160±n/a nM |
|---|
| Citation |  Curtin, ML; Heyman, HR; Clark, RF; Sorensen, BK; Doherty, GA; Hansen, TM; Frey, RR; Sarris, KA; Aguirre, AL; Shrestha, A; Tu, N; Woller, K; Pliushchev, MA; Sweis, RF; Cheng, M; Wilsbacher, JL; Kovar, PJ; Guo, J; Cheng, D; Longenecker, KL; Raich, D; Korepanova, AV; Soni, NB; Algire, MA; Richardson, PL; Marin, VL; Badagnani, I; Vasudevan, A; Buchanan, FG; Maag, D; Chiang, GG; Tse, C; Michaelides, MR SAR and characterization of non-substrate isoindoline urea inhibitors of nicotinamide phosphoribosyltransferase (NAMPT). Bioorg Med Chem Lett27:3317-3325 (2017) [PubMed] Article Curtin, ML; Heyman, HR; Clark, RF; Sorensen, BK; Doherty, GA; Hansen, TM; Frey, RR; Sarris, KA; Aguirre, AL; Shrestha, A; Tu, N; Woller, K; Pliushchev, MA; Sweis, RF; Cheng, M; Wilsbacher, JL; Kovar, PJ; Guo, J; Cheng, D; Longenecker, KL; Raich, D; Korepanova, AV; Soni, NB; Algire, MA; Richardson, PL; Marin, VL; Badagnani, I; Vasudevan, A; Buchanan, FG; Maag, D; Chiang, GG; Tse, C; Michaelides, MR SAR and characterization of non-substrate isoindoline urea inhibitors of nicotinamide phosphoribosyltransferase (NAMPT). Bioorg Med Chem Lett27:3317-3325 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nicotinamide phosphoribosyltransferase |
|---|
| Name: | Nicotinamide phosphoribosyltransferase |
|---|
| Synonyms: | NAMPT | NAMPT_HUMAN | NAmPRTase | Nicotinamide phosphoribosyltransferase | Nicotinamide phosphoribosyltransferase (NAMPT) | PBEF | PBEF1 | Pre-B-cell colony-enhancing factor 1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55524.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P43490 |
|---|
| Residue: | 491 |
|---|
| Sequence: | MNPAAEAEFNILLATDSYKVTHYKQYPPNTSKVYSYFECREKKTENSKLRKVKYEETVFY
GLQYILNKYLKGKVVTKEKIQEAKDVYKEHFQDDVFNEKGWNYILEKYDGHLPIEIKAVP
EGFVIPRGNVLFTVENTDPECYWLTNWIETILVQSWYPITVATNSREQKKILAKYLLETS
GNLDGLEYKLHDFGYRGVSSQETAGIGASAHLVNFKGTDTVAGLALIKKYYGTKDPVPGY
SVPAAEHSTITAWGKDHEKDAFEHIVTQFSSVPVSVVSDSYDIYNACEKIWGEDLRHLIV
SRSTQAPLIIRPDSGNPLDTVLKVLEILGKKFPVTENSKGYKLLPPYLRVIQGDGVDINT
LQEIVEGMKQKMWSIENIAFGSGGGLLQKLTRDLLNCSFKCSYVVTNGLGINVFKDPVAD
PNKRSKKGRLSLHRTPAGNFVTLEEGKGDLEEYGQDLLHTVFKNGKVTKSYSFDEIRKNA
QLNIELEAAHH
|
|
|
|---|
| BDBM50254186 |
|---|
| n/a |
|---|
| Name | BDBM50254186 |
|---|
| Synonyms: | CHEMBL4099778 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H29N3O2 |
|---|
| Mol. Mass. | 379.4953 |
|---|
| SMILES | CN(CCC(C)(C)C)C(=O)c1ccc(NC(=O)N2Cc3ccccc3C2)cc1 |
|---|
| Structure | 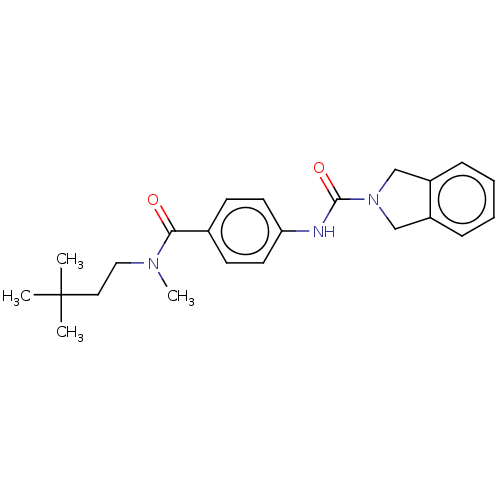
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Curtin, ML; Heyman, HR; Clark, RF; Sorensen, BK; Doherty, GA; Hansen, TM; Frey, RR; Sarris, KA; Aguirre, AL; Shrestha, A; Tu, N; Woller, K; Pliushchev, MA; Sweis, RF; Cheng, M; Wilsbacher, JL; Kovar, PJ; Guo, J; Cheng, D; Longenecker, KL; Raich, D; Korepanova, AV; Soni, NB; Algire, MA; Richardson, PL; Marin, VL; Badagnani, I; Vasudevan, A; Buchanan, FG; Maag, D; Chiang, GG; Tse, C; Michaelides, MR SAR and characterization of non-substrate isoindoline urea inhibitors of nicotinamide phosphoribosyltransferase (NAMPT). Bioorg Med Chem Lett27:3317-3325 (2017) [PubMed] Article
Curtin, ML; Heyman, HR; Clark, RF; Sorensen, BK; Doherty, GA; Hansen, TM; Frey, RR; Sarris, KA; Aguirre, AL; Shrestha, A; Tu, N; Woller, K; Pliushchev, MA; Sweis, RF; Cheng, M; Wilsbacher, JL; Kovar, PJ; Guo, J; Cheng, D; Longenecker, KL; Raich, D; Korepanova, AV; Soni, NB; Algire, MA; Richardson, PL; Marin, VL; Badagnani, I; Vasudevan, A; Buchanan, FG; Maag, D; Chiang, GG; Tse, C; Michaelides, MR SAR and characterization of non-substrate isoindoline urea inhibitors of nicotinamide phosphoribosyltransferase (NAMPT). Bioorg Med Chem Lett27:3317-3325 (2017) [PubMed] Article