

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50012477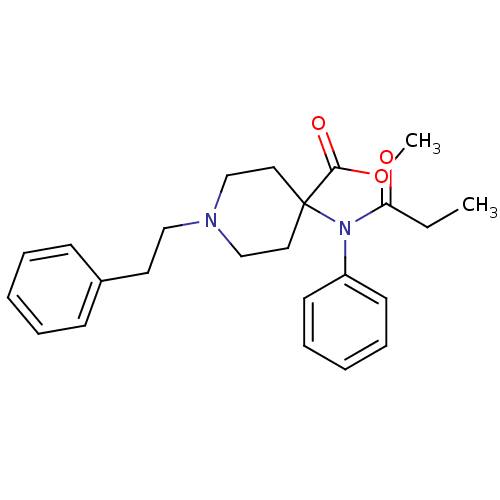 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes | Bioorg Med Chem 22: 4581-6 (2014) Article DOI: 10.1016/j.bmc.2014.07.033 BindingDB Entry DOI: 10.7270/Q2P270WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198754 (CHEMBL3924888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198760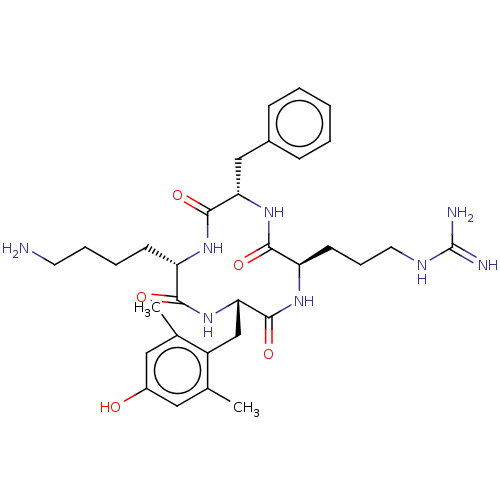 (CHEMBL3897031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198758 (CHEMBL3908315) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50019149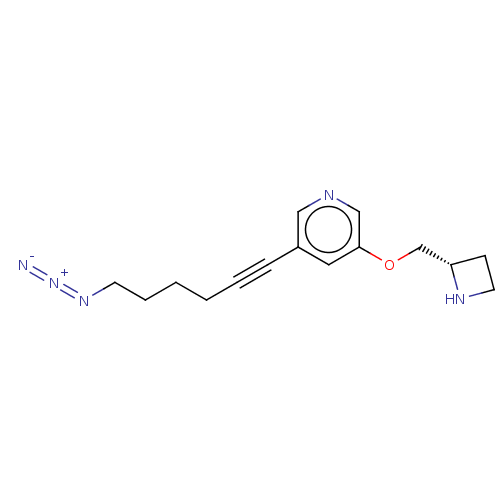 (CHEMBL3289076) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha4beta2 nAChR expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 24: 2954-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.036 BindingDB Entry DOI: 10.7270/Q27P910H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50019144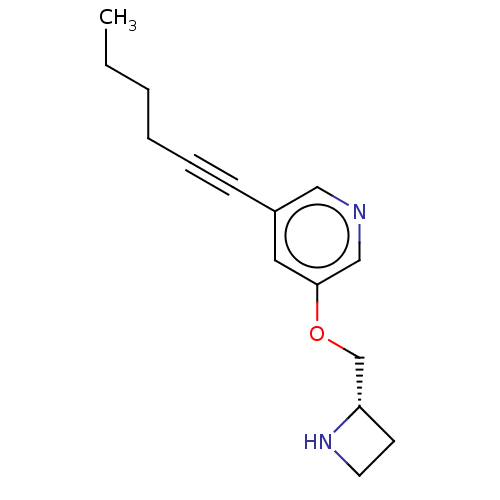 (CHEMBL3289071) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha4beta2 nAChR expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 24: 2954-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.036 BindingDB Entry DOI: 10.7270/Q27P910H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233601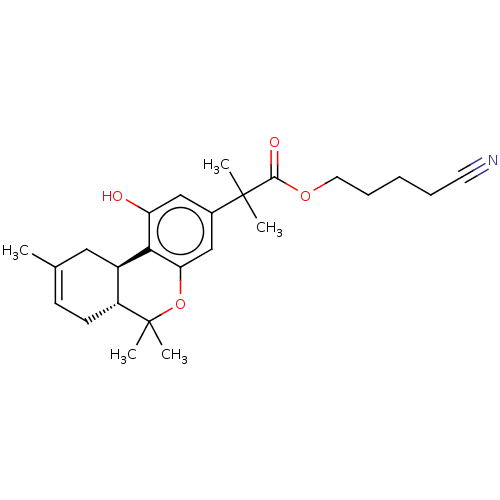 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198755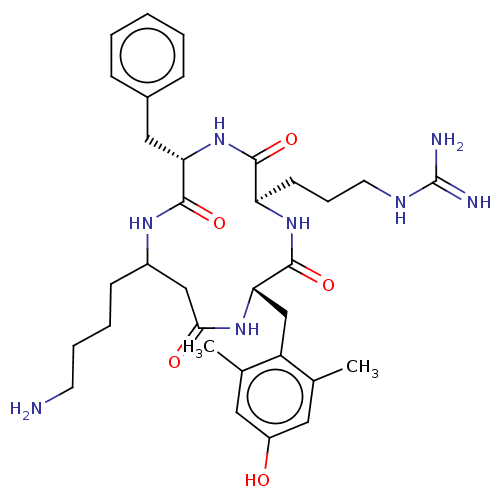 (CHEMBL3979449) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198757 (CHEMBL363142) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599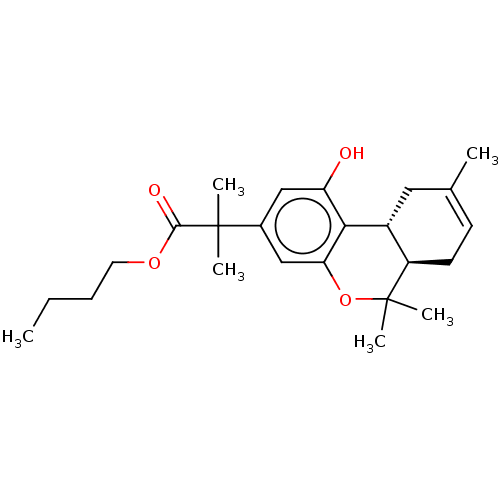 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233619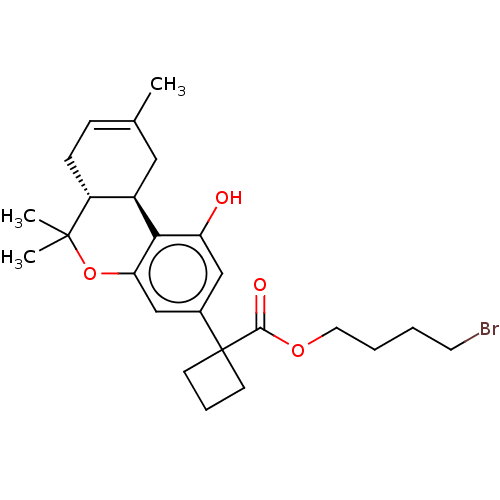 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987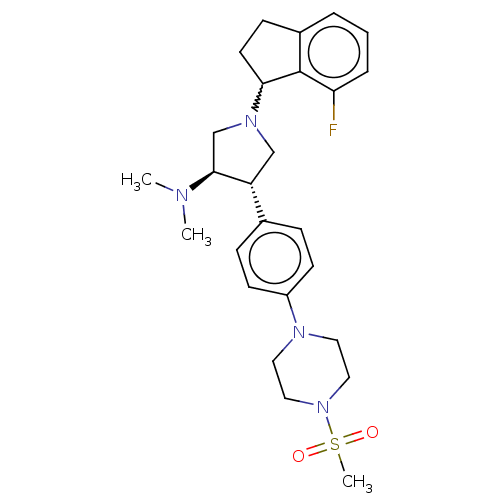 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50314880 (CHEMBL1089542 | N-(3-(guanidinomethyl)benzyl)-N-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.448 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | J Med Chem 53: 2875-81 (2010) Article DOI: 10.1021/jm9019068 BindingDB Entry DOI: 10.7270/Q21G0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50219076 ((6aR-trans)-3-(1-heptylcyclopropyl)-6a,7,10,10a-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain synaptosomal membrane | J Med Chem 50: 4048-60 (2007) Article DOI: 10.1021/jm070121a BindingDB Entry DOI: 10.7270/Q21N80VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50019147 (CHEMBL3289074) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha4beta2 nAChR expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 24: 2954-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.036 BindingDB Entry DOI: 10.7270/Q27P910H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50019145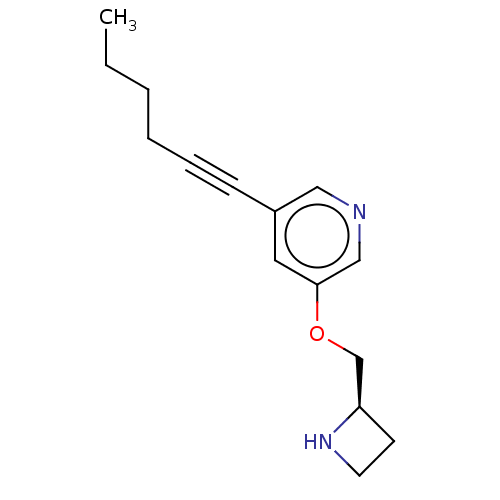 (CHEMBL3289072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha4beta2 nAChR expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 24: 2954-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.036 BindingDB Entry DOI: 10.7270/Q27P910H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233602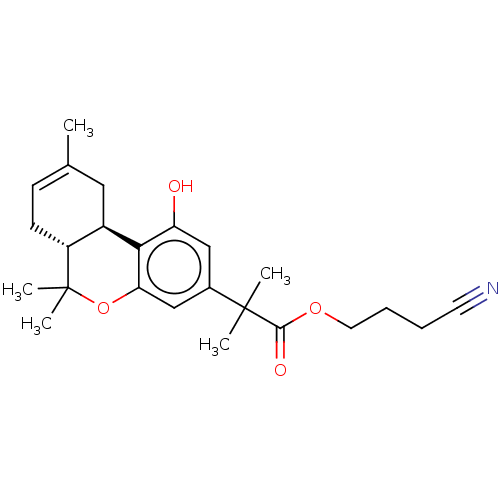 (CHEMBL4071389 | US10882838, Example 1.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233622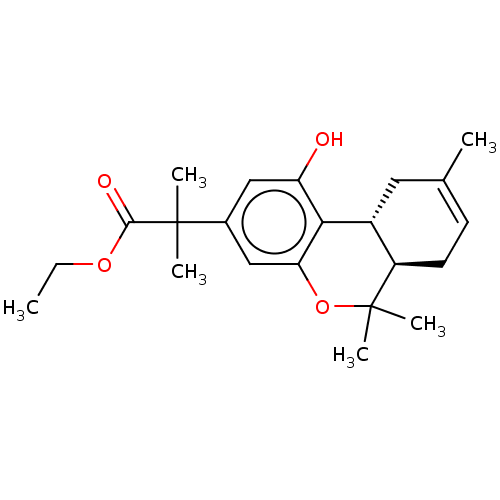 (CHEMBL4073011 | US10882838, Example 1.35) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50314880 (CHEMBL1089542 | N-(3-(guanidinomethyl)benzyl)-N-(1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.536 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane | J Med Chem 53: 2875-81 (2010) Article DOI: 10.1021/jm9019068 BindingDB Entry DOI: 10.7270/Q21G0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50495106 (CHEMBL3104358 | US10882838, Example 1.16) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233608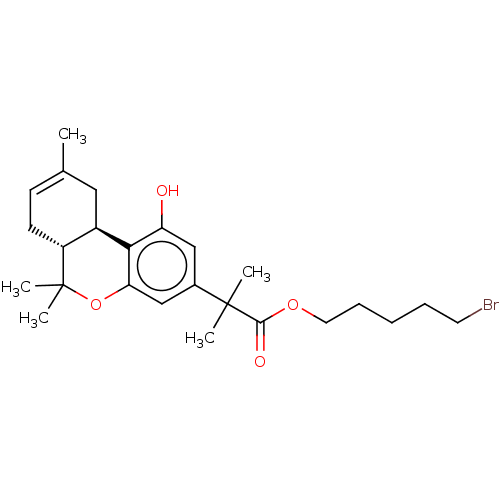 (CHEMBL4094603) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233612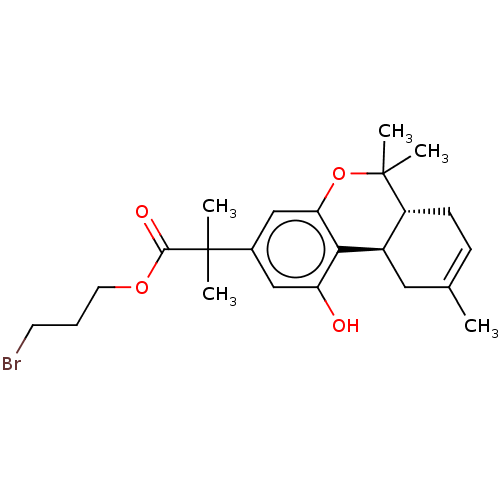 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233619 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233605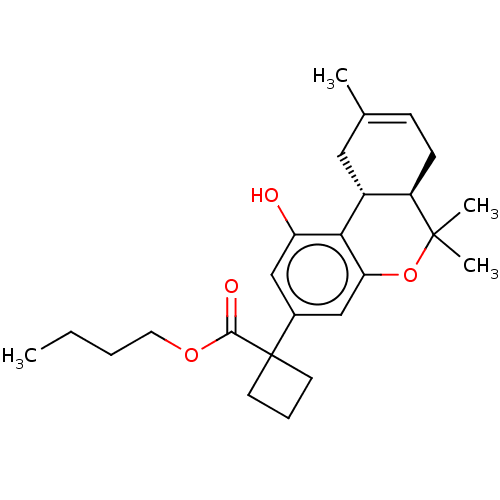 (CHEMBL3104362 | US10882838, Example 1.20) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233601 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233605 (CHEMBL3104362 | US10882838, Example 1.20) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50219078 ((6aR-trans)-3-[1-[(1Z)-1-hexenyl]cyclopentyl]-6a,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain synaptosomal membrane | J Med Chem 50: 4048-60 (2007) Article DOI: 10.1021/jm070121a BindingDB Entry DOI: 10.7270/Q21N80VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198754 (CHEMBL3924888) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198755 (CHEMBL3979449) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.786 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233602 (CHEMBL4071389 | US10882838, Example 1.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233609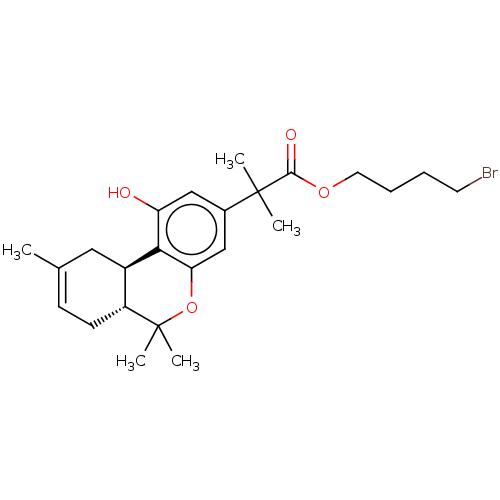 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233612 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198754 (CHEMBL3924888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.807 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233609 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233604 (CHEMBL4063822 | US10882838, Example 1.19) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233601 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50219072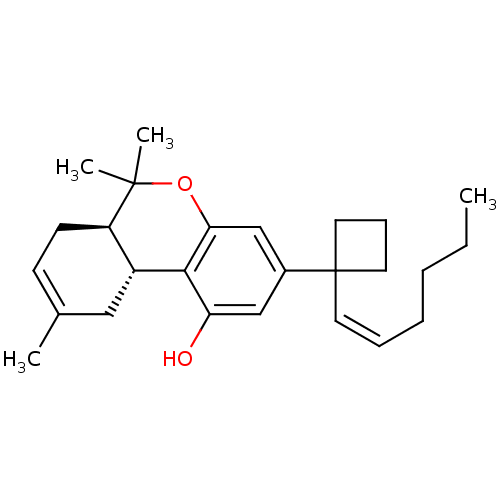 ((6aR-trans)-3-[1-[(1Z)-1-hexenyl]cyclobutyl]-6a,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain synaptosomal membrane | J Med Chem 50: 4048-60 (2007) Article DOI: 10.1021/jm070121a BindingDB Entry DOI: 10.7270/Q21N80VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177016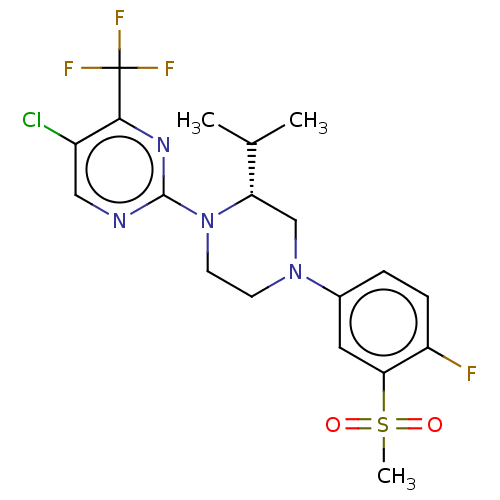 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198752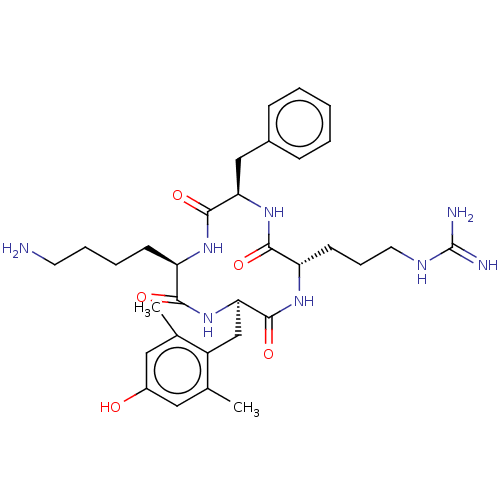 (CHEMBL3976694) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217112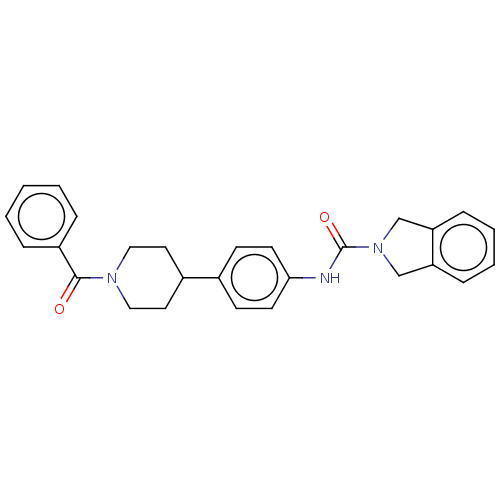 (US9302989, 407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192752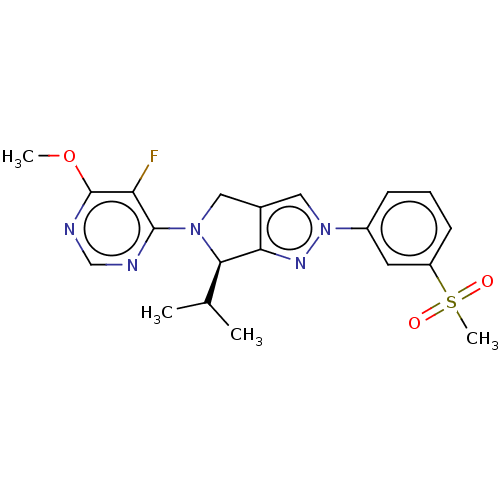 (CHEMBL3905741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223986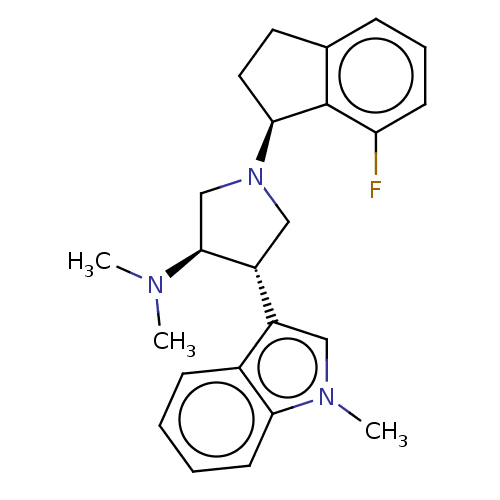 ((3R,4S)-1-[(1S)-7-fluoroindan-1-yl]-N,N-dimethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012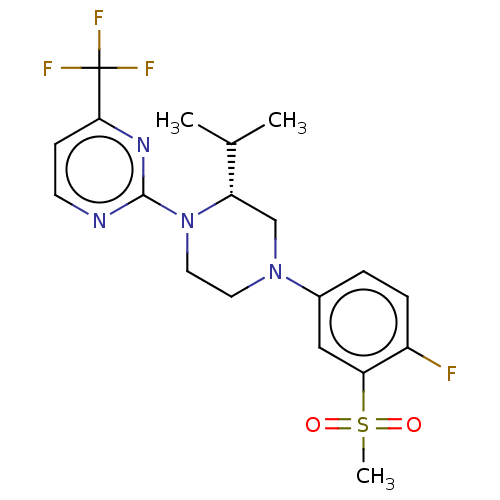 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7078 total ) | Next | Last >> |