| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50455078 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1753846 (CHEMBL4188606) |
|---|
| IC50 | >20000±n/a nM |
|---|
| Citation |  Ruf, S; Hallur, MS; Anchan, NK; Swamy, IN; Murugesan, KR; Sarkar, S; Narasimhulu, LK; Putta, VPRK; Shaik, S; Chandrasekar, DV; Mane, VS; Kadnur, SV; Suresh, J; Bhamidipati, RK; Singh, M; Burri, RR; Kristam, R; Schreuder, H; Czech, J; Rudolph, C; Marker, A; Langer, T; Mullangi, R; Yura, T; Gosu, R; Kannt, A; Dhakshinamoorthy, S; Rajagopal, S Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg Med Chem Lett28:922-925 (2018) [PubMed] Article Ruf, S; Hallur, MS; Anchan, NK; Swamy, IN; Murugesan, KR; Sarkar, S; Narasimhulu, LK; Putta, VPRK; Shaik, S; Chandrasekar, DV; Mane, VS; Kadnur, SV; Suresh, J; Bhamidipati, RK; Singh, M; Burri, RR; Kristam, R; Schreuder, H; Czech, J; Rudolph, C; Marker, A; Langer, T; Mullangi, R; Yura, T; Gosu, R; Kannt, A; Dhakshinamoorthy, S; Rajagopal, S Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg Med Chem Lett28:922-925 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50455078 |
|---|
| n/a |
|---|
| Name | BDBM50455078 |
|---|
| Synonyms: | CHEMBL4218848 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C7H9N3O |
|---|
| Mol. Mass. | 151.1659 |
|---|
| SMILES | CNc1ccc(cn1)C(N)=O |
|---|
| Structure | 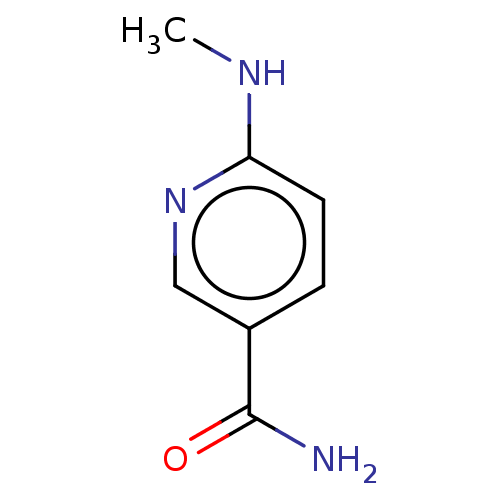
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ruf, S; Hallur, MS; Anchan, NK; Swamy, IN; Murugesan, KR; Sarkar, S; Narasimhulu, LK; Putta, VPRK; Shaik, S; Chandrasekar, DV; Mane, VS; Kadnur, SV; Suresh, J; Bhamidipati, RK; Singh, M; Burri, RR; Kristam, R; Schreuder, H; Czech, J; Rudolph, C; Marker, A; Langer, T; Mullangi, R; Yura, T; Gosu, R; Kannt, A; Dhakshinamoorthy, S; Rajagopal, S Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg Med Chem Lett28:922-925 (2018) [PubMed] Article
Ruf, S; Hallur, MS; Anchan, NK; Swamy, IN; Murugesan, KR; Sarkar, S; Narasimhulu, LK; Putta, VPRK; Shaik, S; Chandrasekar, DV; Mane, VS; Kadnur, SV; Suresh, J; Bhamidipati, RK; Singh, M; Burri, RR; Kristam, R; Schreuder, H; Czech, J; Rudolph, C; Marker, A; Langer, T; Mullangi, R; Yura, T; Gosu, R; Kannt, A; Dhakshinamoorthy, S; Rajagopal, S Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg Med Chem Lett28:922-925 (2018) [PubMed] Article