

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM15339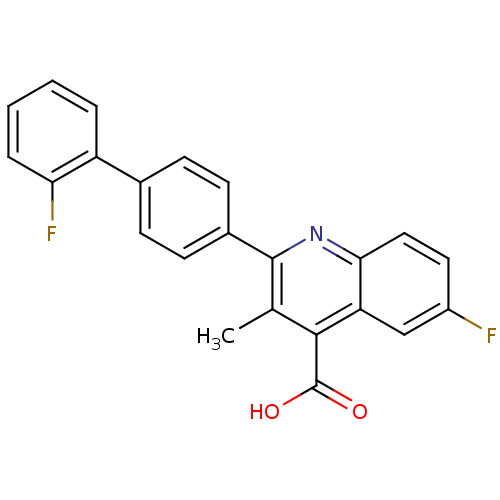 (6-fluoro-2-[4-(2-fluorophenyl)phenyl]-3-methyl-qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455078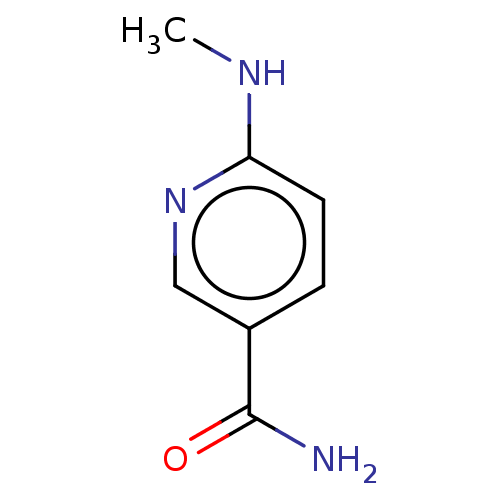 (CHEMBL4218848) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of NNMT in human U2OS cells assessed as reduction in MNA production after 24 hrs by LC-MS/MS analysis | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576030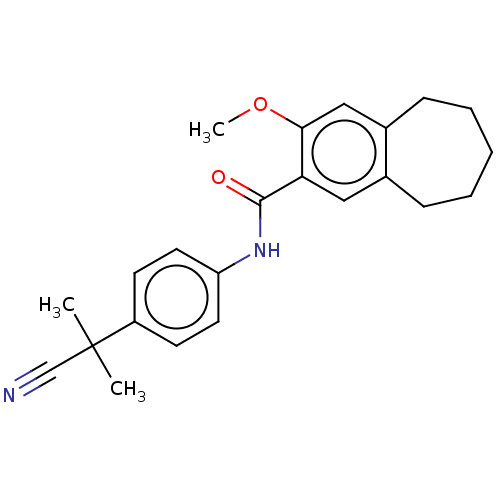 (CHEMBL4860581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455078 (CHEMBL4218848) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455085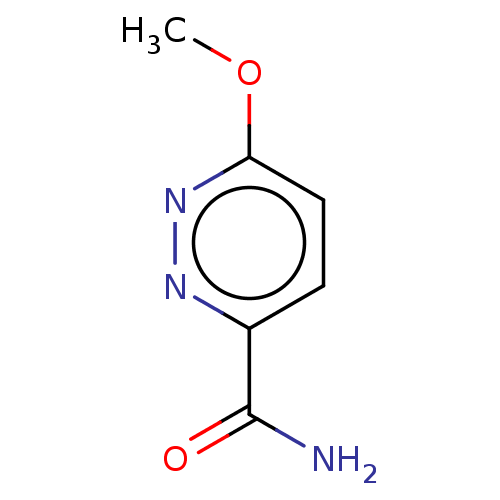 (CHEMBL4217817) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455092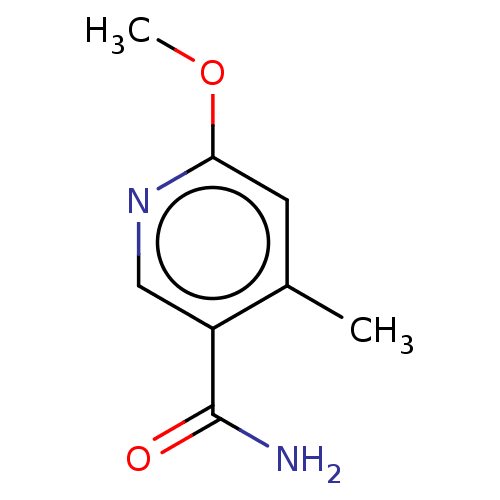 (CHEMBL4218043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 588 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455087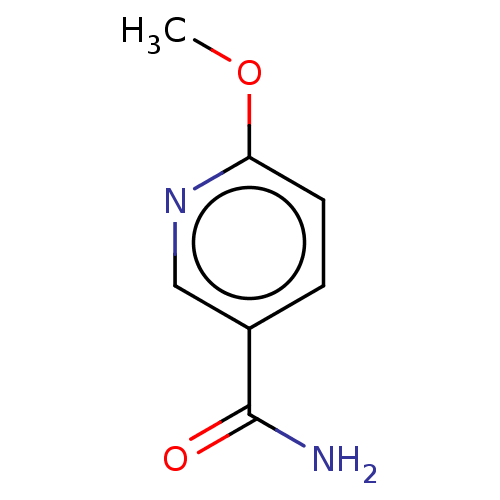 (CHEMBL4207500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 922 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50455078 (CHEMBL4218848) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of NNMT in mouse 3T3L1 cells assessed as reduction in MNA production after 24 hrs by LC-MS/MS analysis | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455095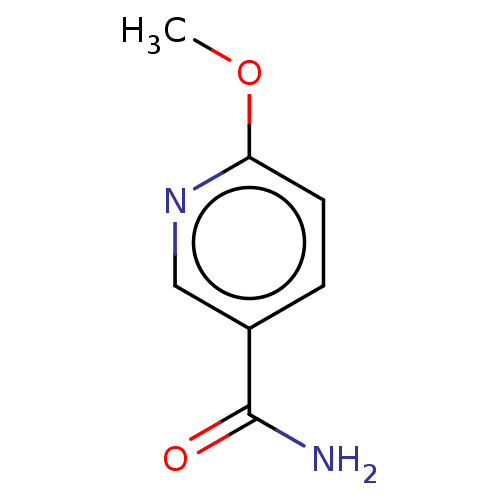 (CHEMBL4206972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576027 (CHEMBL4852930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576023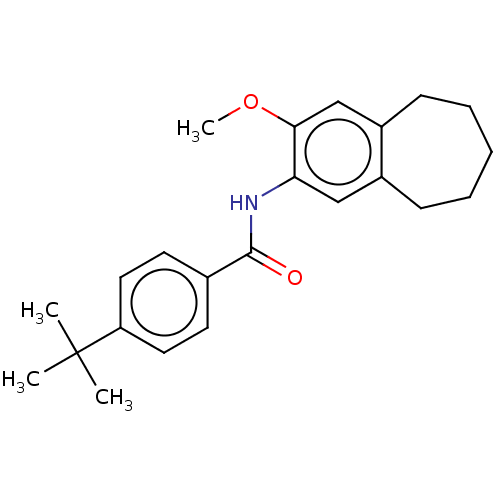 (CHEMBL4851250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50455078 (CHEMBL4218848) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of mouse NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576022 (CHEMBL1498496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92409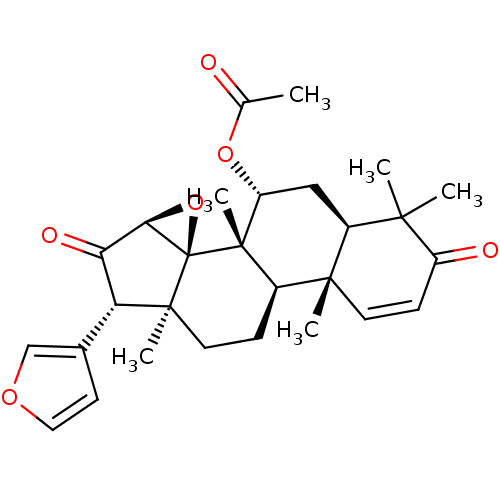 (Epoxyazadiradione) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Council of Scientific and Industrial Research (CSIR) Indian Institute of Chemical Biology | Assay Description The tautomerase activity of MIFs was monitored by following the tautomerization of L-dopachrome methyl ester by the recombinant MIFs in the presence ... | J Biol Chem 287: 24844-61 (2012) Article DOI: 10.1074/jbc.M112.341321 BindingDB Entry DOI: 10.7270/Q2RV0M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50359714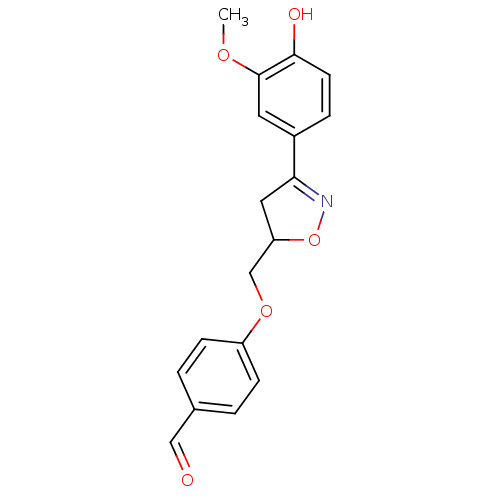 (CHEMBL1927069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of human cloned MIF tautomerase activity expressed in Escherichia coli assessed as conversion of L-dopachrome methyl ester to indolecarbox... | Bioorg Med Chem 19: 7365-73 (2011) Article DOI: 10.1016/j.bmc.2011.10.056 BindingDB Entry DOI: 10.7270/Q2CR5TSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Plasmodium falciparum) | BDBM92409 (Epoxyazadiradione) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Council of Scientific and Industrial Research (CSIR) Indian Institute of Chemical Biology | Assay Description The tautomerase activity of MIFs was monitored by following the tautomerization of L-dopachrome methyl ester by the recombinant MIFs in the presence ... | J Biol Chem 287: 24844-61 (2012) Article DOI: 10.1074/jbc.M112.341321 BindingDB Entry DOI: 10.7270/Q2RV0M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor-like protein (Plasmodium yoelii) | BDBM92409 (Epoxyazadiradione) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Council of Scientific and Industrial Research (CSIR) Indian Institute of Chemical Biology | Assay Description The tautomerase activity of MIFs was monitored by following the tautomerization of L-dopachrome methyl ester by the recombinant MIFs in the presence ... | J Biol Chem 287: 24844-61 (2012) Article DOI: 10.1074/jbc.M112.341321 BindingDB Entry DOI: 10.7270/Q2RV0M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455086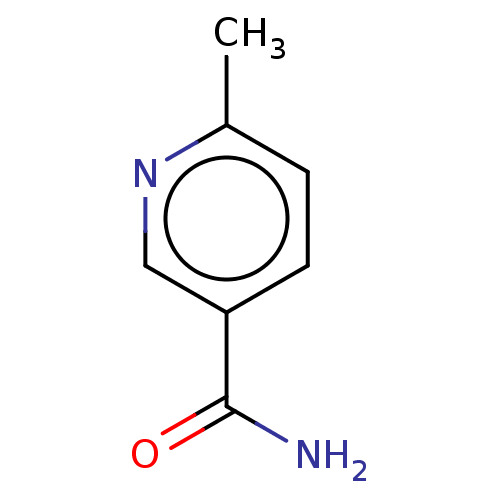 (CHEMBL4204179) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50359718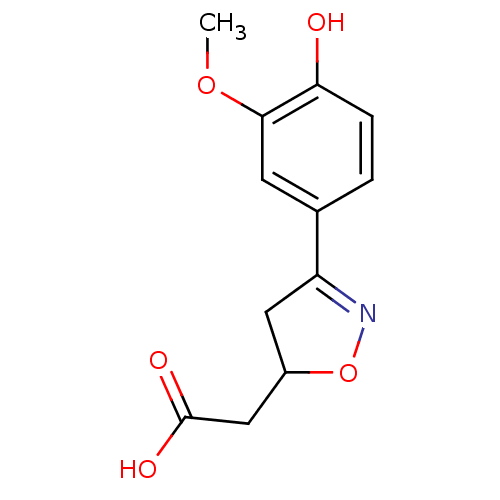 (CHEMBL1927059) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of human cloned MIF tautomerase activity expressed in Escherichia coli assessed as conversion of L-dopachrome methyl ester to indolecarbox... | Bioorg Med Chem 19: 7365-73 (2011) Article DOI: 10.1016/j.bmc.2011.10.056 BindingDB Entry DOI: 10.7270/Q2CR5TSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50359713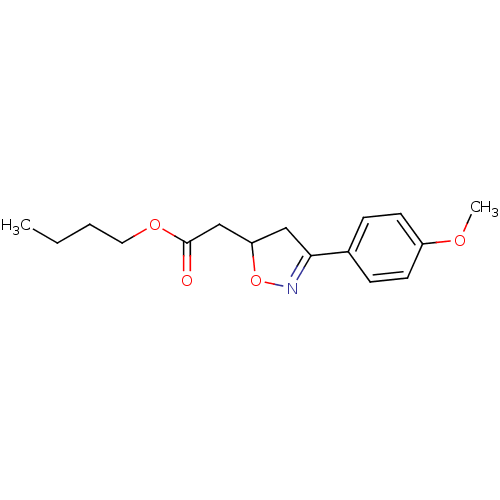 (CHEMBL1927067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of human cloned MIF tautomerase activity expressed in Escherichia coli assessed as conversion of L-dopachrome methyl ester to indolecarbox... | Bioorg Med Chem 19: 7365-73 (2011) Article DOI: 10.1016/j.bmc.2011.10.056 BindingDB Entry DOI: 10.7270/Q2CR5TSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50359716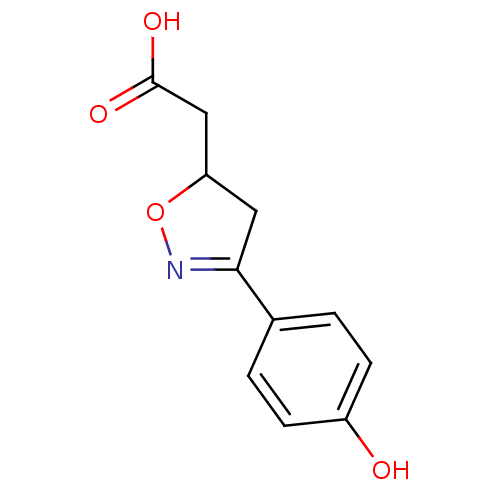 (CHEMBL1927057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of human cloned MIF tautomerase activity expressed in Escherichia coli assessed as conversion of L-dopachrome methyl ester to indolecarbox... | Bioorg Med Chem 19: 7365-73 (2011) Article DOI: 10.1016/j.bmc.2011.10.056 BindingDB Entry DOI: 10.7270/Q2CR5TSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50359717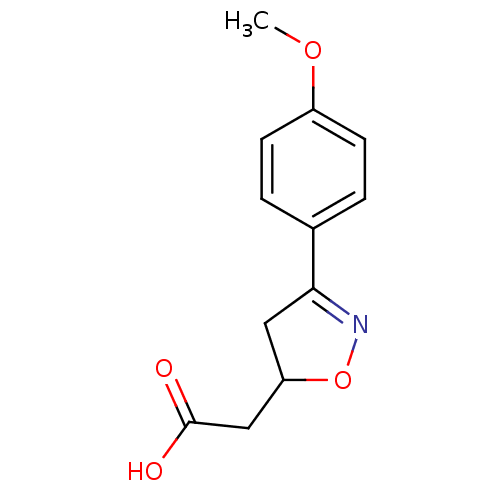 (CHEMBL1927058) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of human cloned MIF tautomerase activity expressed in Escherichia coli assessed as conversion of L-dopachrome methyl ester to indolecarbox... | Bioorg Med Chem 19: 7365-73 (2011) Article DOI: 10.1016/j.bmc.2011.10.056 BindingDB Entry DOI: 10.7270/Q2CR5TSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92412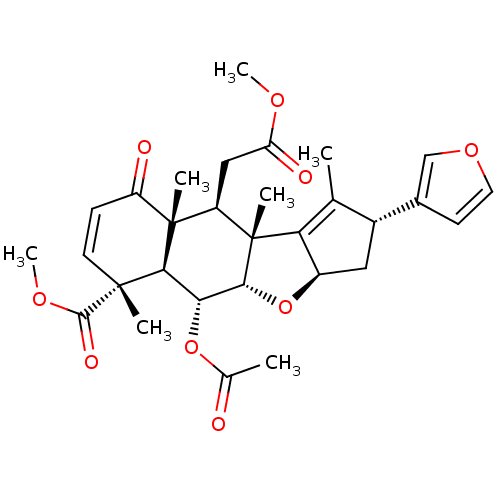 (Nimbin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Council of Scientific and Industrial Research (CSIR) Indian Institute of Chemical Biology | Assay Description The tautomerase activity of MIFs was monitored by following the tautomerization of L-dopachrome methyl ester by the recombinant MIFs in the presence ... | J Biol Chem 287: 24844-61 (2012) Article DOI: 10.1074/jbc.M112.341321 BindingDB Entry DOI: 10.7270/Q2RV0M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50359712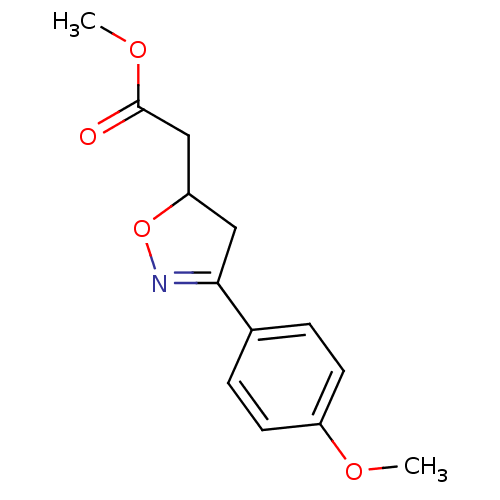 (CHEMBL1927066) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of human cloned MIF tautomerase activity expressed in Escherichia coli assessed as conversion of L-dopachrome methyl ester to indolecarbox... | Bioorg Med Chem 19: 7365-73 (2011) Article DOI: 10.1016/j.bmc.2011.10.056 BindingDB Entry DOI: 10.7270/Q2CR5TSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50455078 (CHEMBL4218848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50455078 (CHEMBL4218848) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50455078 (CHEMBL4218848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50455078 (CHEMBL4218848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor-like protein (Plasmodium yoelii) | BDBM92412 (Nimbin) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Council of Scientific and Industrial Research (CSIR) Indian Institute of Chemical Biology | Assay Description The tautomerase activity of MIFs was monitored by following the tautomerization of L-dopachrome methyl ester by the recombinant MIFs in the presence ... | J Biol Chem 287: 24844-61 (2012) Article DOI: 10.1074/jbc.M112.341321 BindingDB Entry DOI: 10.7270/Q2RV0M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Plasmodium falciparum) | BDBM92412 (Nimbin) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Council of Scientific and Industrial Research (CSIR) Indian Institute of Chemical Biology | Assay Description The tautomerase activity of MIFs was monitored by following the tautomerization of L-dopachrome methyl ester by the recombinant MIFs in the presence ... | J Biol Chem 287: 24844-61 (2012) Article DOI: 10.1074/jbc.M112.341321 BindingDB Entry DOI: 10.7270/Q2RV0M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576029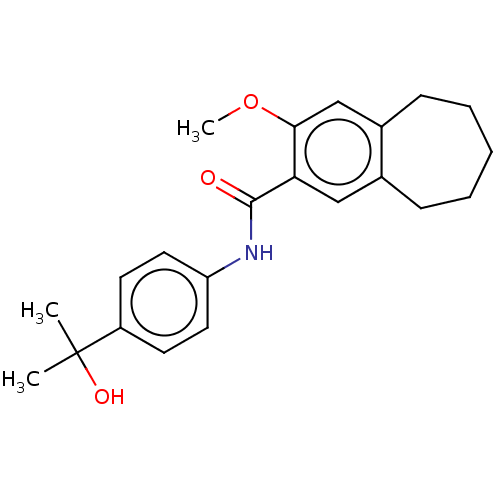 (CHEMBL4858433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50359719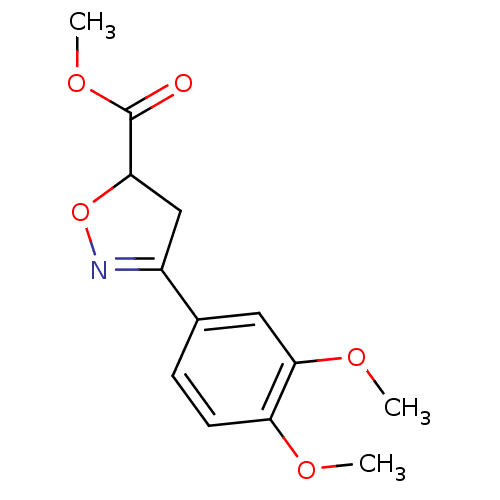 (CHEMBL1927060) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of human cloned MIF tautomerase activity expressed in Escherichia coli assessed as conversion of L-dopachrome methyl ester to indolecarbox... | Bioorg Med Chem 19: 7365-73 (2011) Article DOI: 10.1016/j.bmc.2011.10.056 BindingDB Entry DOI: 10.7270/Q2CR5TSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455093 (CHEBI:27410 | CHEMBL3542314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455091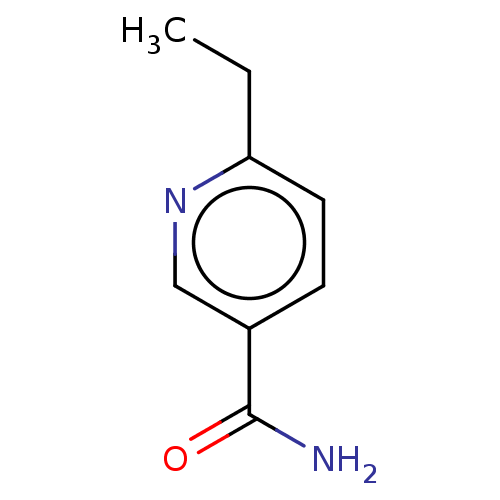 (CHEMBL4216237) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455090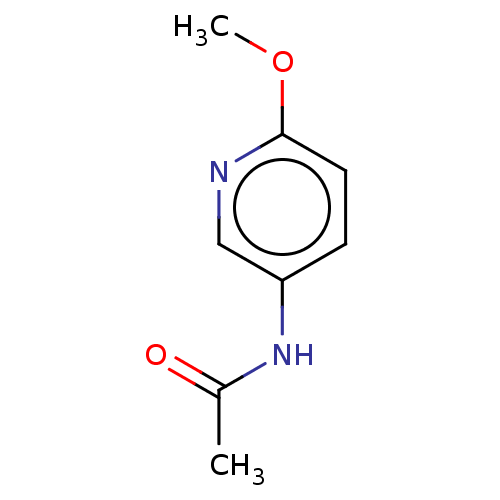 (CHEMBL4202617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455089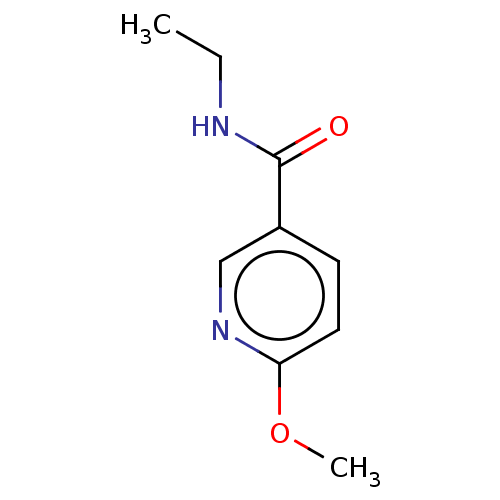 (CHEMBL4209022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455088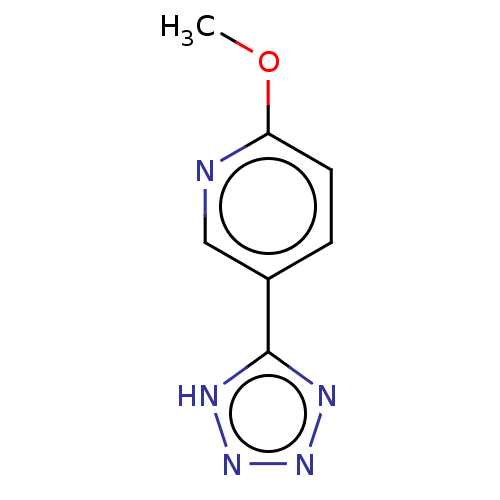 (CHEMBL4206735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50340090 (4-methoxybenzamide | CHEMBL449635) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455084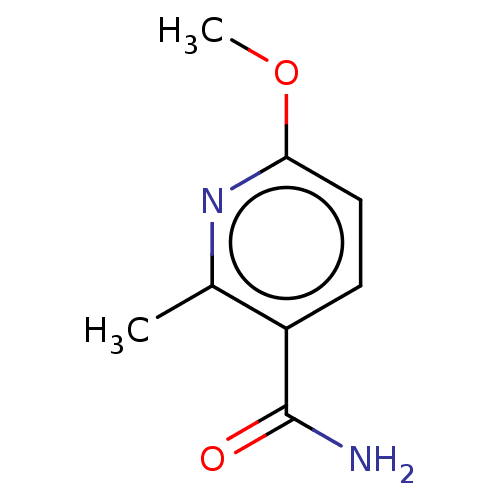 (CHEMBL4214098) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576032 (CHEMBL4869858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455082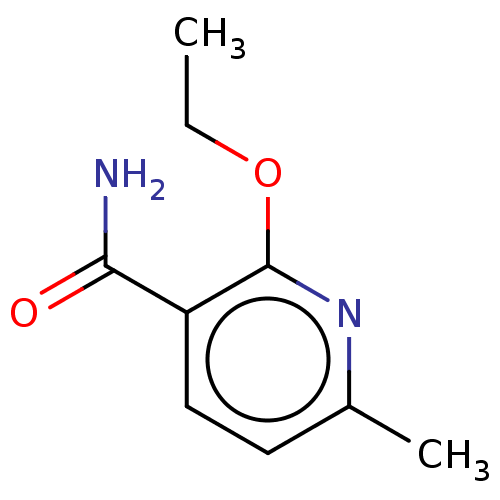 (CHEMBL4215561) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455081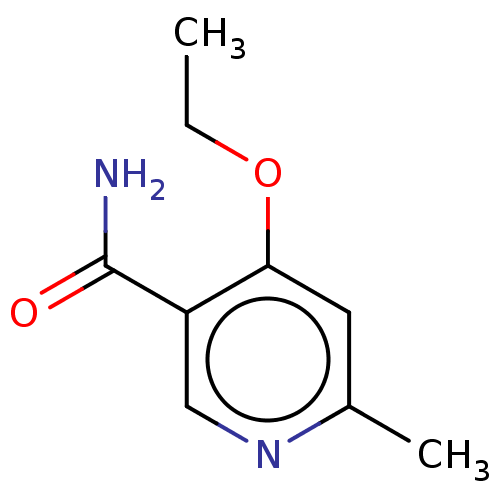 (CHEMBL4209724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455080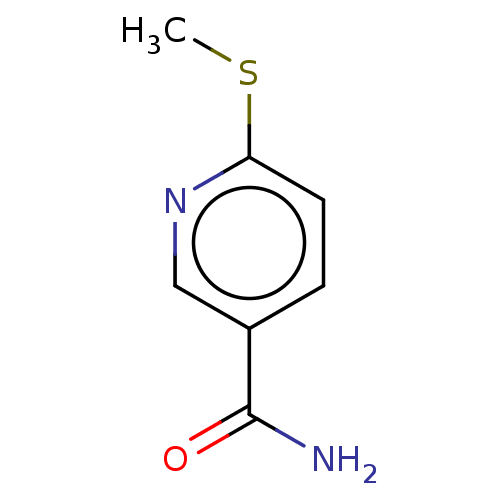 (CHEMBL4207893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455079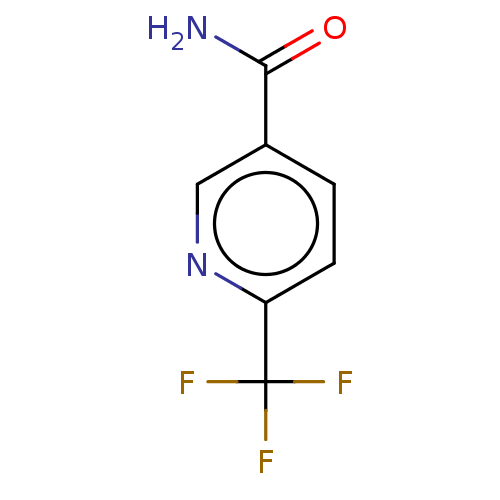 (CHEMBL4210945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM228815 (5-MeO-PZA) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455077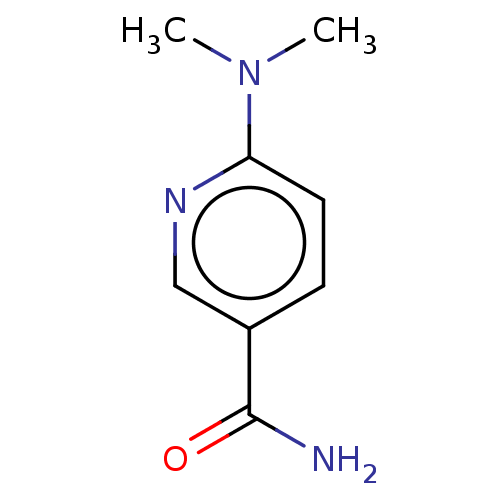 (CHEMBL1997189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50455083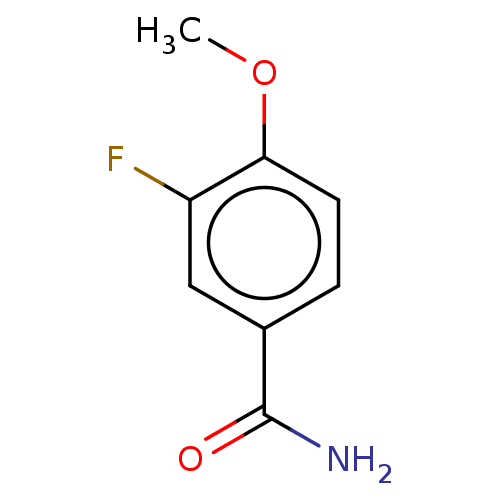 (CHEMBL4218838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NNMT at 30 uM preincubated for 30 mins followed by nicotinamide substrate and SAM addition measured after 60 mins by fluorescence... | Bioorg Med Chem Lett 28: 922-925 (2018) Article DOI: 10.1016/j.bmcl.2018.01.058 BindingDB Entry DOI: 10.7270/Q22Z184C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576031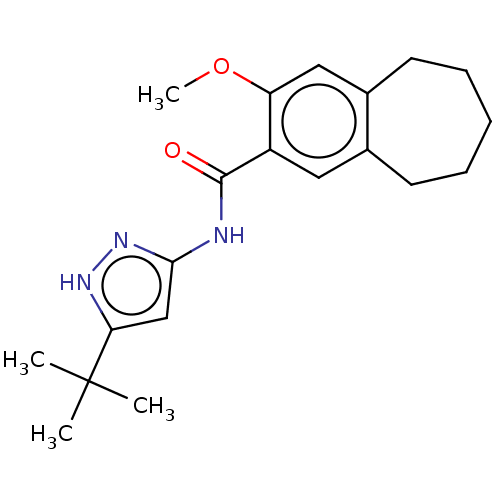 (CHEMBL4874245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576028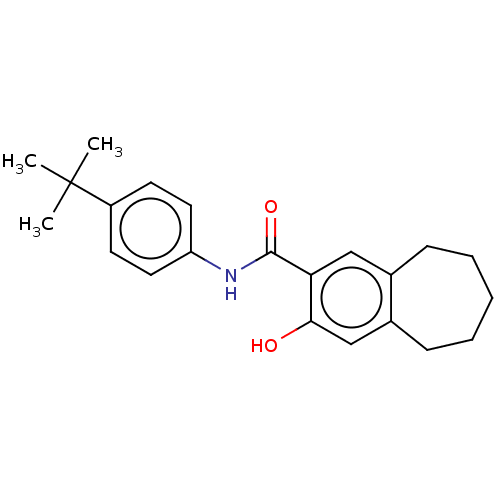 (CHEMBL4849065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50576026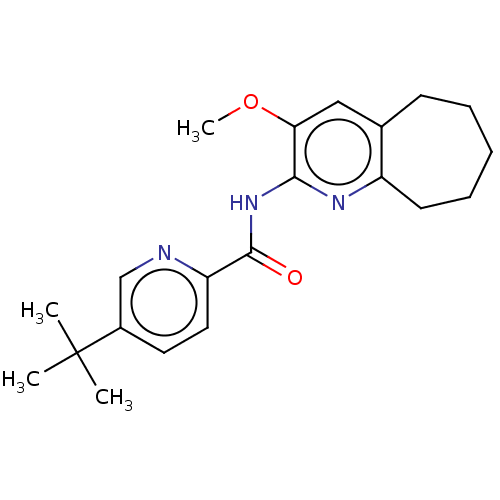 (CHEMBL4876364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02183 BindingDB Entry DOI: 10.7270/Q2W3814Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |