| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prothrombin |
|---|
| Ligand | BDBM50455296 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1754119 (CHEMBL4188879) |
|---|
| IC50 | 4900±n/a nM |
|---|
| Citation |  Afosah, DK; Verespy, S; Al-Horani, RA; Boothello, RS; Karuturi, R; Desai, UR A small group of sulfated benzofurans induces steady-state submaximal inhibition of thrombin. Bioorg Med Chem Lett28:1101-1105 (2018) [PubMed] Article Afosah, DK; Verespy, S; Al-Horani, RA; Boothello, RS; Karuturi, R; Desai, UR A small group of sulfated benzofurans induces steady-state submaximal inhibition of thrombin. Bioorg Med Chem Lett28:1101-1105 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prothrombin |
|---|
| Name: | Prothrombin |
|---|
| Synonyms: | Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 70029.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00734 |
|---|
| Residue: | 622 |
|---|
| Sequence: | MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLEREC
VEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHV
NITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQE
CSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASA
QAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETG
DGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYI
DGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTEN
DLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHP
VCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDST
RIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKY
GFYTHVFRLKKWIQKVIDQFGE
|
|
|
|---|
| BDBM50455296 |
|---|
| n/a |
|---|
| Name | BDBM50455296 |
|---|
| Synonyms: | CHEMBL4202710 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C32H29NaO13S |
|---|
| Mol. Mass. | 676.62 |
|---|
| SMILES | [Na;v0+].[#6]-[#6]-[#6]-[#8]-[#6](=O)-c1c(-[#6]-[#8]-c2cc3c(-[#6](=O)-[#8]-[#6]-c4ccccc4)c(-[#6])oc3cc2-[#8]-[#6])oc2cc(-[#8]-[#6])c(-[#8]S([#8-])(=O)=O)cc12 |
|---|
| Structure | 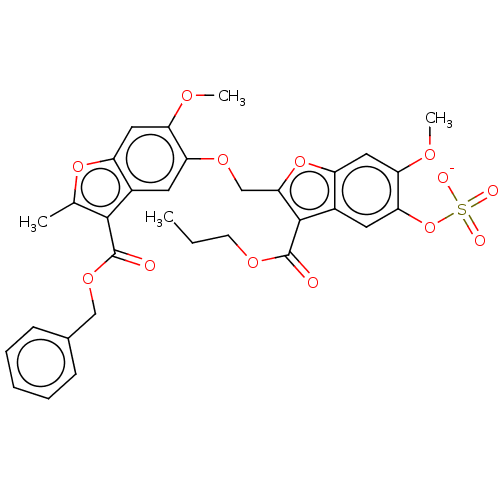
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Afosah, DK; Verespy, S; Al-Horani, RA; Boothello, RS; Karuturi, R; Desai, UR A small group of sulfated benzofurans induces steady-state submaximal inhibition of thrombin. Bioorg Med Chem Lett28:1101-1105 (2018) [PubMed] Article
Afosah, DK; Verespy, S; Al-Horani, RA; Boothello, RS; Karuturi, R; Desai, UR A small group of sulfated benzofurans induces steady-state submaximal inhibition of thrombin. Bioorg Med Chem Lett28:1101-1105 (2018) [PubMed] Article